Related Research Articles

Hydrocodone, sold under the brand name Zohydro ER, among others, is an opioid used to treat severe pain of a prolonged duration, if other measures are not sufficient. It is also used as a cough suppressant in adults. It is taken by mouth. Typically it is sold as the combinations acetaminophen/hydrocodone or ibuprofen/hydrocodone. By itself it is sold in a long-acting formulation.
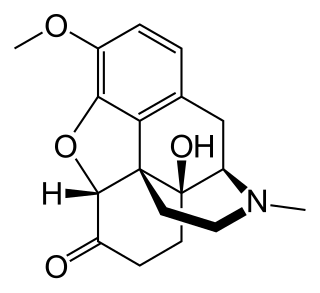
Oxycodone, sold under the brand name OxyContin among others, is an opioid medication used for treatment of moderate to severe pain, and a common drug of abuse. It is usually taken by mouth, and is available in immediate-release and controlled-release formulations. Onset of pain relief typically begins within fifteen minutes and lasts for up to six hours with the immediate-release formulation. In the United Kingdom, it is available by injection. Combination products are also available with paracetamol (acetaminophen), ibuprofen, naloxone, and aspirin.

The term narcotic originally referred medically to any psychoactive compound with numbing or paralysing properties. In the United States, it has since become associated with opiates and opioids, commonly morphine and heroin, as well as derivatives of many of the compounds found within raw opium latex. The primary three are morphine, codeine, and thebaine.

Fentanyl, also spelled fentanil, is an opioid used as a pain medication and together with other medications for anesthesia. It is also used as a recreational drug, often mixed with heroin or cocaine. It has a rapid onset and its effects generally last under two hours. Medically, it is used by injection, nasal spray, skin patch, or absorbed through the cheek (transmucosal) as a lozenge or tablet.
Oxycodone/aspirin is a combination drug marketed by Endo Pharmaceuticals. It is a tablet containing a mixture of 325 mg of aspirin and 4.8355 mg of oxycodone HCl ; it is an opioid/non-opioid combination used to treat moderate to moderately severe pain. The safety of the combination during pregnancy has not been established, although aspirin is generally contraindicated during pregnancy, and the drug has been placed in pregnancy category D. Inactive ingredients include D&C Yellow 10, FD&C Yellow 6, microcrystalline cellulose, and corn starch. Percodan was first marketed by DuPont Pharmaceuticals and prescribed in the United States in 1950. Once a widely prescribed painkiller, it has largely been replaced by alternative oxycodone compounds containing paracetamol (acetaminophen) instead of aspirin, such as Percocet.
Oxycodone/paracetamol, sold under the brand name Percocet among others, is a combination of the opioid oxycodone with paracetamol (acetaminophen), used to treat moderate to severe short-term pain.
ATC code N02Analgesics is a therapeutic subgroup of the Anatomical Therapeutic Chemical Classification System, a system of alphanumeric codes developed by the World Health Organization (WHO) for the classification of drugs and other medical products. Subgroup N02 is part of the anatomical group N Nervous system.

Phenyltoloxamine is an antihistamine with sedative and analgesic effects. It is available in combination with other drugs such as paracetamol (acetominophen).
The Standard for the Uniform Scheduling of Medicines and Poisons (SUSMP) is an Australian legislative instrument produced by the Therapeutic Goods Administration (TGA). Before 2010, it was known as the Standard for the Uniform Scheduling of Drugs and Poisons (SUSDP). The SUSMP classifies drugs and poisons into different Schedules signifying the degree of control recommended to be exercised over their availability to the public. As of 2020, the most recent version is the Poisons Standard July 2020.
Cold water extraction is the process whereby a substance is extracted from a mixture via cold water. It is a type of fractional crystallization.

Hydrocodone/paracetamol, also known as hydrocodone/acetaminophen, is the combination of the pain medications hydrocodone and paracetamol (acetaminophen). It is used to treat moderate to severe pain. It is taken by mouth. Recreational use is common in the United States.
Combined drug intoxication (CDI), or multiple drug intake (MDI), is a cause of death by drug overdose from poly drug use, often implicated in polysubstance dependence.

Fluanisone is a typical antipsychotic and sedative of the butyrophenone chemical class. It is used in the treatment of schizophrenia and mania. It is also a component of the injectable veterinary formulation fentanyl/fluanisone (Hypnorm) where it is used for rodent analgesia during short surgical procedures.

Oxycodone/naloxone, sold under the trade name Targin among others, is a combination pain medication. It is available as modified-release tablets and is taken by mouth.
The drug combination morphine/naltrexone is an opioid combination pain medication developed by King Pharmaceuticals for use in moderate to severe pain. The active ingredients are morphine sulfate and naltrexone hydrochloride; morphine being an opioid receptor agonist and naltrexone an opioid receptor antagonist. It is a schedule 2 controlled substance, and is intended for long-term pain caused by malignancy or where lower tiers of the pain management ladder have already been exhausted, and where medications such as oxycodone would otherwise have been indicated.
Rocky Mountain Poison & Drug Safety (RMPDS), formerly Rocky Mountain Poison and Drug Center (RMPDC), is a department within Denver Health Medical Center, an integrated health care organization based in Denver, Colorado.

Hydrocodone/ibuprofen' (INNs), sold under the brand name Vicoprofen, is a fixed-dose combination analgesic medication used in short-term therapy to relieve severe pain. Vicoprofen combines the analgesic and antitussive properties of hydrocodone with the analgesic, anti-inflammatory, and antipyretic properties of ibuprofen. In contrast to hydrocodone/acetaminophen combination analgesics such as Vicodin, this hydrocodone/ibuprofen avoids some of the liver toxicity which may occur from acetaminophen, but still presents significant dangers in hydrocodone overdose, namely respiratory depression. Vicoprofen is supplied in a fixed dose combination tablet which contains hydrocodone bitartrate, USP 7.5 mg with ibuprofen, USP 200 mg. Additional strengths of generic Vicoprofen are now available, in combinations of 5 mg/200 mg and 10 mg/200 mg respectively.
Hydrocodone/aspirin (INNs) is an oral combination drug formulation of the opioid analgesic hydrocodone and the nonsteroidal anti-inflammatory drug (NSAID) aspirin that is used in the treatment of chronic and acute pain. It is sold under brand names including Alor 5/500, Azdone, Damason-P, Lortab ASA, and Panasal 5/500.
Oxycodone/ibuprofen is an oral combination drug formulation of the opioid analgesic oxycodone and the nonsteroidal anti-inflammatory drug (NSAID) ibuprofen that is used in the treatment of chronic and acute pain. This particular drug is supplied in a fixed dose combination tablet which contains Oxycodone Hydrochloride, USP 5 mg with Ibuprofen, USP 400 mg.