Related Research Articles

In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid chemical compound with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.

Hornblende is a complex inosilicate series of minerals. It is not a recognized mineral in its own right, but the name is used as a general or field term, to refer to a dark amphibole. Hornblende minerals are common in igneous and metamorphic rocks.

Amphibole is a group of inosilicate minerals, forming prism or needlelike crystals, composed of double chain SiO
4 tetrahedra, linked at the vertices and generally containing ions of iron and/or magnesium in their structures. Its IMA symbol is Amp. Amphiboles can be green, black, colorless, white, yellow, blue, or brown. The International Mineralogical Association currently classifies amphiboles as a mineral supergroup, within which are two groups and several subgroups.

Actinolite is an amphibole silicate mineral with the chemical formula Ca2(Mg4.5-2.5Fe2+0.5-2.5)Si8O22(OH)2.

Forsterite (Mg2SiO4; commonly abbreviated as Fo; also known as white olivine) is the magnesium-rich end-member of the olivine solid solution series. It is isomorphous with the iron-rich end-member, fayalite. Forsterite crystallizes in the orthorhombic system (space group Pbnm) with cell parameters a 4.75 Å (0.475 nm), b 10.20 Å (1.020 nm) and c 5.98 Å (0.598 nm).

Tremolite is a member of the amphibole group of silicate minerals with composition: Ca2(Mg5.0-4.5Fe2+0.0-0.5)Si8O22(OH)2. Tremolite forms by metamorphism of sediments rich in dolomite and quartz. Tremolite forms a series with actinolite and ferro-actinolite. Pure magnesium tremolite is creamy white, but the color grades to dark green with increasing iron content. It has a hardness on Mohs scale of 5 to 6. Nephrite, one of the two minerals of the gemstone jade, is a green variety of tremolite.

Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust.

Riebeckite is a sodium-rich member of the amphibole group of silicate minerals, chemical formula Na2(Fe2+3Fe3+2)Si8O22(OH)2. It forms a solid solution series with magnesioriebeckite. It crystallizes in the monoclinic system, usually as long prismatic crystals showing a diamond-shaped cross section, but also in fibrous, bladed, acicular, columnar, and radiating forms. Its Mohs hardness is 5.0–6.0, and its specific gravity is 3.0–3.4. Cleavage is perfect, two directions in the shape of a diamond; fracture is uneven, splintery. It is often translucent to nearly opaque.

The chlorites are the group of phyllosilicate minerals common in low-grade metamorphic rocks and in altered igneous rocks. Greenschist, formed by metamorphism of basalt or other low-silica volcanic rock, typically contains significant amounts of chlorite.

Cummingtonite is a metamorphic amphibole with the chemical composition (Mg,Fe2+
)
2(Mg,Fe2+
)
5Si
8O
22(OH)
2, magnesium iron silicate hydroxide.

Anthophyllite is an amphibole mineral: ☐Mg2Mg5Si8O22(OH)2 (☐ is for a vacancy, a point defect in the crystal structure), magnesium iron inosilicate hydroxide. Anthophyllite is polymorphic with cummingtonite. Some forms of anthophyllite are lamellar or fibrous and are classed as asbestos. The name is derived from the Latin word anthophyllum, meaning clove, an allusion to the most common color of the mineral. The Anthophyllite crystal is characterized by its perfect cleavage along directions 126 degrees and 54 degrees.

The endmember hornblende tschermakite (☐Ca2(Mg3Al2)(Si6Al2)O22(OH)2) is a calcium rich monoclinic amphibole mineral. It is frequently synthesized along with its ternary solid solution series members tremolite and cummingtonite so that the thermodynamic properties of its assemblage can be applied to solving other solid solution series from a variety of amphibole minerals.

Gedrite is a crystal belonging to the orthorhombic ferromagnesian subgroup of the amphibole supergroup of the double chain inosilicate minerals with the ideal chemical formula Mg2(Mg3Al2)(Si6Al2)O22(OH)2.

Dollaseite-(Ce) is a sorosilicate end-member epidote rare-earth mineral which was discovered by Per Geijer (1927) in the Ostanmossa mine, Norberg district, Sweden. Dollaseite-(Ce), although not very well known, is part of a broad epidote group of minerals which are primarily silicates, the most abundant type of minerals on earth. Dollaseite-(Ce) forms as dark-brown subhedral crystals primarily in Swedish mines. With the ideal chemical formula, CaREE3+
Mg
2AlSi
3O
11,(OH)F, dollaseite-(Ce) can be partially identified by its content of the rare earth element cerium.
This list gives an overview of the classification of minerals (silicates) and includes mostly International Mineralogical Association (IMA) recognized minerals and its groupings. This list complements the List of minerals recognized by the International Mineralogical Association series of articles and List of minerals. Rocks, ores, mineral mixtures, non-IMA approved minerals and non-named minerals are mostly excluded.

Magnesiohastingsite is a calcium-containing amphibole and a member of the hornblende group. It is an inosilicate (chain silicate) with the formula NaCa2(Mg4Fe3+)(Si6Al2)O22(OH)2 and molar mass 864.69 g. In synthetic magnesiohastingsite it appears that iron occurs both as ferrous iron Fe2+ and as ferric iron Fe3+, but the ideal formula features only ferric iron. It was named in 1928 by Marland P. Billings. The name is for its relationship to hastingsite and its magnesium content. Hastingsite was named for the locality in Dungannon Township, Hastings County, Ontario, Canada.
Mineral alteration refers to the various natural processes that alter a mineral's chemical composition or crystallography.

Hexagonite is the red to pink, lilac to purple manganoan variety of tremolite. A rare amphibole, it can be transparent, translucent, and rarely opaque.
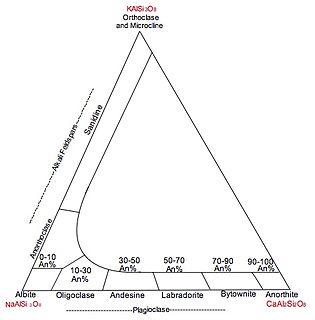
Coupled substitution is the geological process by which two elements simultaneous substitute into a crystal in order to maintain overall electrical neutrality and keep the charge constant. In forming a solid solution series, ionic size is more important than ionic charge, as this can be compensated for elsewhere in the structure.