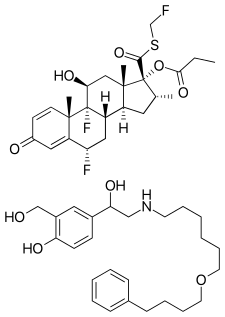
Fluticasone is a manufactured glucocorticoid used to treat nasal symptoms. Both the esters, fluticasone propionate and fluticasone furoate, are also used as topical anti-inflammatories and inhaled corticosteroids, and are used much more commonly in comparison.
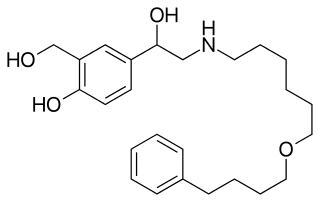
Salmeterol is a long-acting β2 adrenergic receptor agonist (LABA) used in the maintenance and prevention of asthma symptoms and maintenance of chronic obstructive pulmonary disease (COPD) symptoms. Symptoms of bronchospasm include shortness of breath, wheezing, coughing and chest tightness. It is also used to prevent breathing difficulties during exercise.

Budesonide/formoterol, sold under the brand name Symbicort among others, is a fixed-dose combination medication used in the management of asthma or chronic obstructive pulmonary disease (COPD). It contains budesonide, a steroid and formoterol, a long-acting β2-agonist (LABA). The product monograph does not support its use for sudden worsening or treatment of active bronchospasm. However, a 2020 review of the literature does support such use. It is used by breathing in the medication.

Formoterol, also known as eformoterol, is a long-acting β2 agonist (LABA) used as a bronchodilator in the management of asthma and chronic obstructive pulmonary disease (COPD). Formoterol has an extended duration of action compared to short-acting β2 agonists such as salbutamol (albuterol), which are effective for 4 h to 6 h. Formoterol has a relatively rapid onset of action compared to other LABAs, and is effective within 2-3 minutes. The 2022 Global Initiative for Asthma report recommends a combination formoterol/inhaled corticosteroid inhaler as both a preventer and reliever treatment for asthma in adults. In children, a short-acting β2 adrenergic agonist is still recommended.
Beta2-adrenergic agonists, also known as adrenergic β2 receptor agonists, are a class of drugs that act on the β2 adrenergic receptor. Like other β adrenergic agonists, they cause smooth muscle relaxation. β2 adrenergic agonists' effects on smooth muscle cause dilation of bronchial passages, vasodilation in muscle and liver, relaxation of uterine muscle, and release of insulin. They are primarily used to treat asthma and other pulmonary disorders. Bronchodilators are considered an important treatment regime for chronic obstructive pulmonary disease (COPD) and are usually used in combination with short acting medications and long acting medications in a combined inhaler.

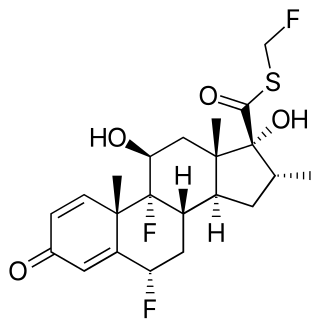
Fluticasone propionate, sold under the brand names Flovent and Flonase among others, is a steroid medication. When inhaled it is used for the long term management of asthma and COPD. In the nose it is used for hay fever and nasal polyps. It can also be used for mouth ulcers. It works by decreasing inflammation.

Bronchoconstriction is the constriction of the airways in the lungs due to the tightening of surrounding smooth muscle, with consequent coughing, wheezing, and shortness of breath.

Long-acting β adrenoceptor agonists (LABAs) are beta-adrenergic agonists usually prescribed for moderate-to-severe persistent asthma and chronic obstructive pulmonary disease (COPD). They are designed to reduce the need for shorter-acting β2 agonists such as salbutamol (albuterol), as they have a duration of action of approximately 12 hours in comparison with the 4-to-6-hour duration of salbutamol, making them candidates for sparing high doses of corticosteroids or treating nocturnal asthma and providing symptomatic improvement in patients with COPD. With the exception of formoterol, long-acting β2 agonists are not recommended for the treatment of acute asthma exacerbations because of their slower onset of action compared to salbutamol. Their long duration of action is due to the addition of a long, lipophilic side-chain that binds to an exosite on adrenergic receptors. This allows the active portion of the molecule to continuously bind and unbind at β2 receptors in the smooth muscle in the lungs.

Mometasone, also known as mometasone y 3 s, is a steroid medication used to treat certain skin conditions, hay fever, and asthma. Specifically it is used to prevent rather than treat asthma attacks. It can be applied to the skin, inhaled, or used in the nose. Mometasone furoate, not mometasone, is used in medical products.
Fluticasone furoate, sold under the brand name Flonase Sensimist among others, is a corticosteroid for the treatment of non-allergic and allergic rhinitis administered by a nasal spray. It is also available as an inhaled corticosteroid to help prevent and control symptoms of asthma. It is derived from cortisol. Unlike fluticasone propionate, which is only approved for children four years and older, fluticasone furoate is approved in children as young as two years of age when used for allergies.

Budesonide, sold under the brand name Pulmicort, among others, is a steroid medication. It is available as an inhaler, nebulization solution, pill, nasal spray, and rectal forms. The inhaled form is used in the long-term management of asthma and chronic obstructive pulmonary disease (COPD). The nasal spray is used for allergic rhinitis and nasal polyps. Modified-release pills or capsules and rectal forms may be used for inflammatory bowel disease including Crohn's disease, ulcerative colitis, and microscopic colitis.

Mometasone/formoterol, sold under the brand name Dulera among others, is a fixed-dose combination medication used in the long-term treatment of asthma. It contains mometasone a steroid and formoterol a long-acting beta agonist. It is only recommended in those for whom an inhaled steroid is not sufficient. It is used by inhalation.

Olodaterol is an ultra-long-acting β adrenoreceptor agonist (ultra-LABA) used as an inhalation for treating people with chronic obstructive pulmonary disease (COPD). It is manufactured by Boehringer Ingelheim.

Vilanterol is an ultra-long-acting β2 adrenoreceptor agonist (ultra-LABA), which was approved in May 2013 in combination with fluticasone furoate for sale as Breo Ellipta by GlaxoSmithKline for the treatment of chronic obstructive pulmonary disease (COPD).. The combination is also approved for the treatment of asthma in Canada, Europe, Japan and New Zealand.

Fluticasone furoate/vilanterol, sold under the brand name Breo Ellipta among others, is a combination medication for the treatment of chronic obstructive pulmonary disease (COPD) and asthma. It contains fluticasone furoate, an inhaled corticosteroid, and vilanterol, an ultra-long-acting β2 agonist (ultra-LABA).

Beclometasone/formoterol/glycopyrronium, sold under the brand name Trimbow among others, is an inhalable fixed-dose combination medication for the treatment of chronic obstructive pulmonary disease (COPD) and asthma. It contains beclometasone dipropionate, formoterol fumarate dihydrate, and glycopyrronium bromide.
Fluticasone furoate/umeclidinium bromide/vilanterol, sold under the brand name Trelegy Ellipta among others, is a fixed-dose combination inhaled medication that is used for the maintenance treatment of chronic obstructive pulmonary disease (COPD). The medications work in different ways: fluticasone furoate is an inhaled corticosteroid (ICS), umeclidinium is a long-acting muscarinic antagonist (LAMA), and vilanterol is a long-acting beta-agonist (LABA).
Indacaterol/mometasone, sold under the brand name Atectura Breezhaler among others, is a fixed-dose combination medication for the treatment of asthma in adults and adolescents twelve years of age and older not adequately controlled with inhaled corticosteroids and inhaled short acting beta2 agonists.
Indacaterol/glycopyrronium bromide/mometasone, sold under the brand name Enerzair Breezhaler among others, is an inhalable fixed-dose combination medication for the treatment of asthma. It contains indacaterol as acetate, glycopyrronium bromide, and mometasone furoate.
Salbutamol/budesonide, sold under the brand name Airsupra, is a fixed-dose combination medication for the treatment of bronchoconstriction and asthma. It is a combination of salbutamol sulfate, a short-acting beta2-adrenergic agonist, and budesonide, an inhaled corticosteroid. It is inhaled using a pressurized metered-dose inhaler.