Related Research Articles
GTPases are a large family of hydrolase enzymes that bind to the nucleotide guanosine triphosphate (GTP) and hydrolyze it to guanosine diphosphate (GDP). The GTP binding and hydrolysis takes place in the highly conserved P-loop "G domain", a protein domain common to many GTPases.

G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their activity is regulated by factors that control their ability to bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP). When they are bound to GTP, they are 'on', and, when they are bound to GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPases.
Small GTPases, also known as small G-proteins, are a family of hydrolase enzymes that can bind and hydrolyze guanosine triphosphate (GTP). They are a type of G-protein found in the cytosol that are homologous to the alpha subunit of heterotrimeric G-proteins, but unlike the alpha subunit of G proteins, a small GTPase can function independently as a hydrolase enzyme to bind to and hydrolyze a guanosine triphosphate (GTP) to form guanosine diphosphate (GDP). The best-known members are the Ras GTPases and hence they are sometimes called Ras subfamily GTPases.

Guanine nucleotide exchange factors (GEFs) are proteins or protein domains that activate monomeric GTPases by stimulating the release of guanosine diphosphate (GDP) to allow binding of guanosine triphosphate (GTP). A variety of unrelated structural domains have been shown to exhibit guanine nucleotide exchange activity. Some GEFs can activate multiple GTPases while others are specific to a single GTPase.

Heterotrimeric G protein, also sometimes referred to as the "large" G proteins are membrane-associated G proteins that form a heterotrimeric complex. The biggest non-structural difference between heterotrimeric and monomeric G protein is that heterotrimeric proteins bind to their cell-surface receptors, called G protein-coupled receptors, directly. These G proteins are made up of alpha (α), beta (β) and gamma (γ) subunits. The alpha subunit is attached to either a GTP or GDP, which serves as an on-off switch for the activation of G-protein.

The Gs alpha subunit is a subunit of the heterotrimeric G protein Gs that stimulates the cAMP-dependent pathway by activating adenylyl cyclase. Gsα is a GTPase that functions as a cellular signaling protein. Gsα is the founding member of one of the four families of heterotrimeric G proteins, defined by the alpha subunits they contain: the Gαs family, Gαi/Gαo family, Gαq family, and Gα12/Gα13 family. The Gs-family has only two members: the other member is Golf, named for its predominant expression in the olfactory system. In humans, Gsα is encoded by the GNAS complex locus, while Golfα is encoded by the GNAL gene.

Transforming protein RhoA, also known as Ras homolog family member A (RhoA), is a small GTPase protein in the Rho family of GTPases that in humans is encoded by the RHOA gene. While the effects of RhoA activity are not all well known, it is primarily associated with cytoskeleton regulation, mostly actin stress fibers formation and actomyosin contractility. It acts upon several effectors. Among them, ROCK1 and DIAPH1 are the best described. RhoA, and the other Rho GTPases, are part of a larger family of related proteins known as the Ras superfamily, a family of proteins involved in the regulation and timing of cell division. RhoA is one of the oldest Rho GTPases, with homologues present in the genomes since 1.5 billion years. As a consequence, RhoA is somehow involved in many cellular processes which emerged throughout evolution. RhoA specifically is regarded as a prominent regulatory factor in other functions such as the regulation of cytoskeletal dynamics, transcription, cell cycle progression and cell transformation.
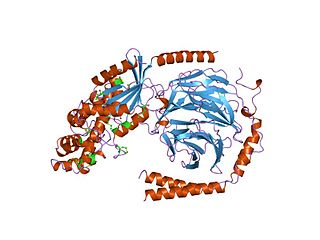
G alpha subunits are one of the three types of subunit of guanine nucleotide binding proteins, which are membrane-associated, heterotrimeric G proteins.

Guanine nucleotide-binding protein G(q) subunit alpha is a protein that in humans is encoded by the GNAQ gene. Together with GNA11, it functions as a Gq alpha subunit.

Regulators of G protein signaling (RGS) are protein structural domains or the proteins that contain these domains, that function to activate the GTPase activity of heterotrimeric G-protein α-subunits.

A-kinase anchor protein 13 is a protein that in humans, is encoded by the AKAP13 gene. This protein is also called AKAP-Lbc because it encodes the lymphocyte blast crisis (Lbc) oncogene, and ARHGEF13/RhoGEF13 because it contains a guanine nucleotide exchange factor (GEF) domain for the RhoA small GTP-binding protein.

Rho guanine nucleotide exchange factor 1 is a protein that in humans is encoded by the ARHGEF1 gene. This protein is also called RhoGEF1 or p115-RhoGEF.

Rho guanine nucleotide exchange factor 11 is a protein that in humans is encoded by the ARHGEF11 gene. This protein is also called RhoGEF11 or PDZ-RhoGEF.

Rho guanine nucleotide exchange factor 12 is a protein that in humans is encoded by the ARHGEF12 gene. This protein is also called RhoGEF12 or Leukemia-associated Rho guanine nucleotide exchange factor (LARG).

RhoG is a small monomeric GTP-binding protein, and is an important component of many intracellular signalling pathways. It is a member of the Rac subfamily of the Rho family of small G proteins and is encoded by the gene RHOG.
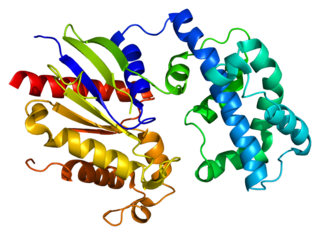
Guanine nucleotide-binding protein subunit alpha-13 is a protein that in humans is encoded by the GNA13 gene.

Guanine nucleotide-binding protein subunit alpha-12 is a protein that in humans is encoded by the GNA12 gene.

The G beta-gamma complex (Gβγ) is a tightly bound dimeric protein complex, composed of one Gβ and one Gγ subunit, and is a component of heterotrimeric G proteins. Heterotrimeric G proteins, also called guanosine nucleotide-binding proteins, consist of three subunits, called alpha, beta, and gamma subunits, or Gα, Gβ, and Gγ. When a G protein-coupled receptor (GPCR) is activated, Gα dissociates from Gβγ, allowing both subunits to perform their respective downstream signaling effects. One of the major functions of Gβγ is the inhibition of the Gα subunit.

GoLoco motif is a protein structural motif. In heterotrimeric G-protein signalling, cell surface receptors (GPCRs) are coupled to membrane-associated heterotrimers comprising a GTP-hydrolyzing subunit G-alpha and a G-beta/G-gamma dimer. The inactive form contains the alpha subunit bound to GDP and complexes with the beta and gamma subunit. When the ligand is associated to the receptor, GDP is displaced from G-alpha and GTP is bound. The GTP/G-alpha complex dissociates from the trimer and associates to an effector until the intrinsic GTPase activity of G-alpha returns the protein to GDP bound form. Reassociation of GDP-bound G-alpha with G-beta/G-gamma dimer terminates the signal. Several mechanisms regulate the signal output at different stage of the G-protein cascade. Two classes of intracellular proteins act as inhibitors of G protein activation: GTPase activating proteins (GAPs), which enhance GTP hydrolysis, and guanine dissociation inhibitors (GDIs), which inhibit GDP dissociation. The GoLoco or G-protein regulatory (GPR) motif found in various G-protein regulators. acts as a GDI on G-alpha(i).

Pleckstrin homology domain containing, family G member 2 (PLEKHG2) is a protein that in humans is encoded by the PLEKHG2 gene. It is sometimes written as ARHGEF42, FLJ00018.
References
- ↑ Dhanasekaran N, Dermott JM (1996). "Signaling by the G12 class of G proteins". Cell. Signal. 8 (4): 235–45. doi:10.1016/0898-6568(96)00048-4. PMID 8842523.
- ↑ Strathmann MP, Simon MI (1991). "G alpha 12 and G alpha 13 subunits define a fourth class of G protein alpha subunits". Proc. Natl. Acad. Sci. U.S.A. 88 (13): 5582–6. Bibcode:1991PNAS...88.5582S. doi: 10.1073/pnas.88.13.5582 . PMC 51921 . PMID 1905812.
- ↑ Gilman, AG (1987). "G proteins: transducers of receptor-generated signals". Annual Review of Biochemistry. 56: 615–649. doi:10.1146/annurev.bi.56.070187.003151. PMID 3113327.
- ↑ Rodbell, M (1995). "Nobel Lecture: Signal transduction: Evolution of an idea". Bioscience Reports. 15 (3): 117–133. doi:10.1007/bf01207453. PMC 1519115 . PMID 7579038. S2CID 11025853.
- ↑ Harhammer R, Nürnberg B, Harteneck C, Leopoldt D, Exner T, Schultz G (1996). "Distinct biochemical properties of the native members of the G12 G-protein subfamily. Characterization of G alpha 12 purified from rat brain". Biochem. J. 319. ( Pt 1): 165–71. doi:10.1042/bj3190165. PMC 1217750 . PMID 8870664.
- ↑ Wang D, Tan YC, Kreitzer GE, Nakai Y, Shan D, Zheng Y, Huang XY (2006). "G proteins G12 and G13 control the dynamic turnover of growth factor-induced dorsal ruffles". J. Biol. Chem. 281 (43): 32660–7. doi: 10.1074/jbc.M604588200 . PMID 16943201.
- ↑ Shan D, Chen L, Wang D, Tan YC, Gu JL, Huang XY (2006). "The G protein G alpha(13) is required for growth factor-induced cell migration". Dev. Cell. 10 (6): 707–18. doi: 10.1016/j.devcel.2006.03.014 . PMID 16740474.