Related Research Articles

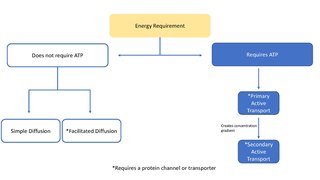
Facilitated diffusion is the process of spontaneous passive transport of molecules or ions across a biological membrane via specific transmembrane integral proteins. Being passive, facilitated transport does not directly require chemical energy from ATP hydrolysis in the transport step itself; rather, molecules and ions move down their concentration gradient according to the principles of diffusion.
In cellular biology, active transport is the movement of molecules or ions across a cell membrane from a region of lower concentration to a region of higher concentration—against the concentration gradient. Active transport requires cellular energy to achieve this movement. There are two types of active transport: primary active transport that uses adenosine triphosphate (ATP), and secondary active transport that uses an electrochemical gradient. This process is in contrast to passive transport, which allows molecules or ions to move down their concentration gradient, from an area of high concentration to an area of low concentration, without energy.

Reuptake is the reabsorption of a neurotransmitter by a neurotransmitter transporter located along the plasma membrane of an axon terminal or glial cell after it has performed its function of transmitting a neural impulse.
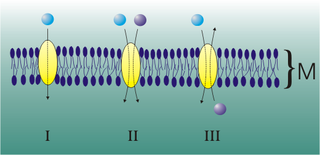
Mediated transport refers to transport mediated by a membrane transport protein. Substances in the human body may be hydrophobic, electrophilic, contain a positively or negatively charge, or have another property. As such there are times when those substances may not be able to pass over the cell membrane using protein-independent movement. The cell membrane is imbedded with many membrane transport proteins that allow such molecules to travel in and out of the cell. There are three types of mediated transporters: uniport, symport, and antiport. Things that can be transported are nutrients, ions, glucose, etc, all depending on the needs of the cell. One example of a uniport mediated transport protein is GLUT1. GLUT1 is a transmembrane protein, which means it spans the entire width of the cell membrane, connecting the extracellular and intracellular region. It is a uniport system because it specifically transports glucose in only one direction, down its concentration gradient across the cell membrane.
A membrane transport protein is a membrane protein involved in the movement of ions, small molecules, and macromolecules, such as another protein, across a biological membrane. Transport proteins are integral transmembrane proteins; that is they exist permanently within and span the membrane across which they transport substances. The proteins may assist in the movement of substances by facilitated diffusion, active transport, osmosis, or reverse diffusion. The two main types of proteins involved in such transport are broadly categorized as either channels or carriers. Examples of channel/carrier proteins include the GLUT 1 uniporter, sodium channels, and potassium channels. The solute carriers and atypical SLCs are secondary active or facilitative transporters in humans. Collectively membrane transporters and channels are known as the transportome. Transportomes govern cellular influx and efflux of not only ions and nutrients but drugs as well.
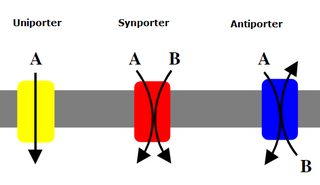
Uniporters, also known as solute carriers or facilitated transporters, are a type of membrane transport protein that passively transports solutes across a cell membrane. It uses facilitated diffusion for the movement of solutes down their concentration gradient from an area of high concentration to an area of low concentration. Unlike active transport, it does not require energy in the form of ATP to function. Uniporters are specialized to carry one specific ion or molecule and can be categorized as either channels or carriers. Facilitated diffusion may occur through three mechanisms: uniport, symport, or antiport. The difference between each mechanism depends on the direction of transport, in which uniport is the only transport not coupled to the transport of another solute.

Cotransporters are a subcategory of membrane transport proteins (transporters) that couple the favorable movement of one molecule with its concentration gradient and unfavorable movement of another molecule against its concentration gradient. They enable coupled or cotransport and include antiporters and symporters. In general, cotransporters consist of two out of the three classes of integral membrane proteins known as transporters that move molecules and ions across biomembranes. Uniporters are also transporters but move only one type of molecule down its concentration gradient and are not classified as cotransporters.

Glucose transporters are a wide group of membrane proteins that facilitate the transport of glucose across the plasma membrane, a process known as facilitated diffusion. Because glucose is a vital source of energy for all life, these transporters are present in all phyla. The GLUT or SLC2A family are a protein family that is found in most mammalian cells. 14 GLUTS are encoded by the human genome. GLUT is a type of uniporter transporter protein.
In biology, an ion transporter is a transmembrane protein that moves ions across a biological membrane to accomplish many different biological functions, including cellular communication, maintaining homeostasis, energy production, etc. There are different types of transporters including pumps, uniporters, antiporters, and symporters. Active transporters or ion pumps are transporters that convert energy from various sources—including adenosine triphosphate (ATP), sunlight, and other redox reactions—to potential energy by pumping an ion up its concentration gradient. This potential energy could then be used by secondary transporters, including ion carriers and ion channels, to drive vital cellular processes, such as ATP synthesis.
Method of glucose uptake differs throughout tissues depending on two factors; the metabolic needs of the tissue and availability of glucose. The two ways in which glucose uptake can take place are facilitated diffusion and secondary active transport. Active transport is the movement of ions or molecules going against the concentration gradient.
Glucose transporter 1, also known as solute carrier family 2, facilitated glucose transporter member 1 (SLC2A1), is a uniporter protein that in humans is encoded by the SLC2A1 gene. GLUT1 facilitates the transport of glucose across the plasma membranes of mammalian cells. This gene encodes a facilitative glucose transporter that is highly expressed in erythrocytes and endothelial cells, including cells of the blood–brain barrier. The encoded protein is found primarily in the cell membrane and on the cell surface, where it can also function as a receptor for human T-cell leukemia virus (HTLV) I and II. GLUT1 accounts for 2 percent of the protein in the plasma membrane of erythrocytes.
Sodium-dependent glucose cotransporters are a family of glucose transporter found in the intestinal mucosa (enterocytes) of the small intestine (SGLT1) and the proximal tubule of the nephron. They contribute to renal glucose reabsorption. In the kidneys, 100% of the filtered glucose in the glomerulus has to be reabsorbed along the nephron. If the plasma glucose concentration is too high (hyperglycemia), glucose passes into the urine (glucosuria) because SGLT are saturated with the filtered glucose.

A symporter is an integral membrane protein that is involved in the transport of two different molecules across the cell membrane in the same direction. The symporter works in the plasma membrane and molecules are transported across the cell membrane at the same time, and is, therefore, a type of cotransporter. The transporter is called a symporter, because the molecules will travel in the same direction in relation to each other. This is in contrast to the antiport transporter. Typically, the ion(s) will move down the electrochemical gradient, allowing the other molecule(s) to move against the concentration gradient. The movement of the ion(s) across the membrane is facilitated diffusion, and is coupled with the active transport of the molecule(s). In symport, two molecule move in a 'similar direction' at the 'same time'. For example, the movement of glucose along with sodium ions. It exploits the uphill movement of other molecules from low to high concentration, which is against the electrochemical gradient for the transport of solute molecules downhill from higher to lower concentration.

Sodium/glucose cotransporter 1 (SGLT1) also known as solute carrier family 5 member 1 is a protein in humans that is encoded by the SLC5A1 gene which encodes the production of the SGLT1 protein to line the absorptive cells in the small intestine and the epithelial cells of the kidney tubules of the nephron for the purpose of glucose uptake into cells. Recently, it has been seen to have functions that can be considered as promising therapeutic target to treat diabetes and obesity. Through the use of the sodium glucose cotransporter 1 protein, cells are able to obtain glucose which is further utilized to make and store energy for the cell.

The major facilitator superfamily (MFS) is a superfamily of membrane transport proteins that facilitate movement of small solutes across cell membranes in response to chemiosmotic gradients.

Lactose permease is a membrane protein which is a member of the major facilitator superfamily. Lactose permease can be classified as a symporter, which uses the proton gradient towards the cell to transport β-galactosides such as lactose in the same direction into the cell.

Members of the Solute:Sodium Symporter (SSS) Family (TC# 2.A.21) catalyze solute:Na+ symport. The SSS family is within the APC Superfamily. The solutes transported may be sugars, amino acids, organo cations such as choline, nucleosides, inositols, vitamins, urea or anions, depending on the system. Members of the SSS family have been identified in bacteria, archaea and eukaryotes. Almost all functionally well-characterized members normally catalyze solute uptake via Na+ symport.

The Escherichia coliAcriflavine resistance encode a multi-drug efflux system that is believed to protect the bacterium against hydrophobic inhibitors. The E. coli AcrB protein is a transporter that is energized by proton-motive force and that shows the widest substrate specificity among all known multidrug pumps, ranging from most of the currently used antibiotics, disinfectants, dyes, and detergents to simple solvents.

Bacterial Leucine Transporter (LeuT) is a bundled twelve alpha helix protein which belongs to the family of transporters that shuttle amino acids in and out of bacterial cells. Specialized in small hydrophobic amino acids such as leucine and alanine, this transporter is powered by the gradient of sodium ions that is normally maintained by healthy cells across their membranes. LeuT acts as a symporter, which means that it links the passage of a sodium ion across the cell membrane with the transport of the amino acid in the same direction. It was first crystallized to understand the inner molecular mechanisms of antidepressant's work since it has a close resemblance with the human neurotransmitter transporters that these drugs block, thus inhibiting the reuptake of chemical messengers across the cell membrane of nerve axons and glial cells.

Major facilitator superfamily domain containing 6 like (MFSD6L) is a protein encoded by the MFSD6L gene in humans. The MFSD6L protein is a transmembrane protein that is part of the major facilitator superfamily (MFS) that uses chemiosmotic gradients to facilitate the transport of small solutes across cell membranes.
References
1. Henderson, P. J. F., Giddens, R. A. and Jones-Mortimer, M. C. (1977) The transport of galactose, glucose and their molecular analogues by Escherichia coli K12. Biochem. J. 162, 309-320.
2. McDonald, T. P., Walmsley, A. R. and Henderson, P. J. F. (1997) Asparagine 394 in putative helix 11 of the galactose-H+ symport protein (GalP) from Escherichia coli is associated with the internal binding site for cytochalasin B and sugar. J. Biol. Chem. 272, 15189-15199.
3. McDonald, T. P. and Henderson, P. J. F. (2001) Cysteine residues in the D-galactose-H+ symport protein of Escherichia coli: effects of mutagenesis on transport, reaction with N-ethylmaleimide and antibiotic binding. BioChem. J. 353, 709-717.
4. Zheng, H., Taraska, J., Merz, A. J. and Gonen, T. (2010) The Prototypical H+/Galactose Symporter GalP Assembles into Functional Trimers. J. Mol. Biol. 396(3), 593-601.
5. El Qaidi, S., Allemand, J.O., and Plumbridge, J. (2009). Repression of galP, the galactose transporter in Escherichia coli, requires the specific regulator of N-acetylglucosamine metabolism. Molecular Microbiology 71: 146-157.
6. Hase, C. C., Fedorova, N. D., Galperin, M. Y., and Dibrov, P. A. (2001). Sodium ion cycle in bacterial pathogens: evidence from cross-genome comparisons. Microbiology and Molecular Biology Reviews Vol. 65, No. 3: 353-370.
7. Hernandez-Montalvo, V., Martinez, A., Hernandez-Chavez, G., Bolivar, F., Valle, F., and Gosset, G. (2003). Expression of galP and glk in an Escherichia coli PTS mutant restores glucose transport and increases glycolytic flux to fermentation products. Biotechnology and Bioengineering, Vol. 83, No. 6: 687-694.
8. Jung, H. (2002). The sodium/substrate symporter family: structural and functional features. Federation of European Biochemical Societies 529: 73-77.
9. Moller, T., Franch, T., Udesen, C., Gerdes, K., and Valentin-Hansen, P. (2002). Spot 42 RNA mediates discoordinate expression of the E. coli galactose operon. Genes and Development 16: 1696-1706.
10. Sarker, R. I., Ogawa, W., Shimamoto, T., Shimamoto, T., and Tsuchiya, T. (1996). Primary structure and properties of Vibrio parahaemolyticus. Journal of Bacteriology, Vol. 179, No. 5: 1805-1808.
11. Semsey, S., Krishna, S., Sneppen, K., and Adhya, S. (2007). Signal integration in the galactose network of Escherichia coli. Molecular Microbiology, 65: 465-476.
12. Olson, A.L., and Pessin, J.E. (1996). Structure, function, and regulation of the mammalian facilitative glucose transporter gene family. Annu Rev Nutr 16:235-56.
13. White, D. (2007). The Physiology and Biochemistry of Prokaryotes, 3rd Edition. Oxford University Press, New York.
14. Schweizer, H. (2011). Homeostasis. Lecture. 7 March 2011.