Galenic formulation deals with the principles of preparing and compounding medicines in order to optimize their absorption. Galenic formulation is named after Claudius Galen, a 2nd Century AD Greek physician, who codified the preparation of drugs using multiple ingredients. Today, galenic formulation is part of pharmaceutical formulation. The pharmaceutical formulation of a medicine affects the pharmacokinetics, pharmacodynamics and safety profile of a drug.

Pharmacology is a branch of medicine, biology and pharmaceutical sciences concerned with drug or medication action, where a drug may be defined as any artificial, natural, or endogenous molecule which exerts a biochemical or physiological effect on the cell, tissue, organ, or organism. More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function. If substances have medicinal properties, they are considered pharmaceuticals.
A chemical patent, pharmaceutical patent or drug patent is a patent for an invention in the chemical or pharmaceuticals industry. Strictly speaking, in most jurisdictions, there are essentially no differences between the legal requirements to obtain a patent for an invention in the chemical or pharmaceutical fields, in comparison to obtaining a patent in the other fields, such as in the mechanical field. A chemical patent or a pharmaceutical patent is therefore not a sui generis right, i.e. a special legal type of patent.
An excipient is a substance formulated alongside the active ingredient of a medication, included for the purpose of long-term stabilization, bulking up solid formulations that contain potent active ingredients in small amounts, or to confer a therapeutic enhancement on the active ingredient in the final dosage form, such as facilitating drug absorption, reducing viscosity, or enhancing solubility. Excipients can also be useful in the manufacturing process, to aid in the handling of the active substance concerns such as by facilitating powder flowability or non-stick properties, in addition to aiding in vitro stability such as prevention of denaturation or aggregation over the expected shelf life. The selection of appropriate excipients also depends upon the route of administration and the dosage form, as well as the active ingredient and other factors. A comprehensive classification system based on structure-property-application relationships has been proposed for excipients used in parenteral medications.
Pharmaceutics is the discipline of pharmacy that deals with the process of turning a new chemical entity (NCE) or old drugs into a medication to be used safely and effectively by patients. It is also called the science of dosage form design. There are many chemicals with pharmacological properties, but need special measures to help them achieve therapeutically relevant amounts at their sites of action. Pharmaceutics helps relate the formulation of drugs to their delivery and disposition in the body. Pharmaceutics deals with the formulation of a pure drug substance into a dosage form. Branches of pharmaceutics include:

Bioequivalence is a term in pharmacokinetics used to assess the expected in vivo biological equivalence of two proprietary preparations of a drug. If two products are said to be bioequivalent it means that they would be expected to be, for all intents and purposes, the same.
An active ingredient is the ingredient in a pharmaceutical drug or pesticide that is biologically active. The similar terms active pharmaceutical ingredient and bulk active are also used in medicine, and the term active substance may be used for natural products. Some medication products may contain more than one active ingredient. The traditional word for the active pharmaceutical agent is pharmacon or pharmakon which originally denoted a magical substance or drug.
Dr. Reddy's Laboratories is an Indian multinational pharmaceutical company located in Hyderabad, Telangana, India. The company was founded by Kallam Anji Reddy, who previously worked in the mentor institute Indian Drugs and Pharmaceuticals Limited. Dr. Reddy's manufactures and markets a wide range of pharmaceuticals in India and overseas. The company has over 190 medications, 60 active pharmaceutical ingredients (APIs) for drug manufacture, diagnostic kits, critical care, and biotechnology products.

Oxybutynin, sold as under the brand names Ditropan among others, is a medication used to treat overactive bladder. It works similar to tolterodine, Darifenacin, and Solifenacin. While used for bed wetting in children, evidence to support this use is poor. It is taken by mouth or applied to the skin.
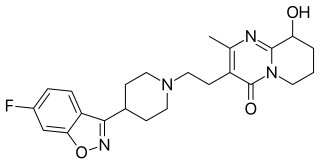
Paliperidone, sold under the trade name Invega among others, is an atypical antipsychotic. It is mainly used to treat schizophrenia and schizoaffective disorder.
Pharmaceutical formulation, in pharmaceutics, is the process in which different chemical substances, including the active drug, are combined to produce a final medicinal product. The word formulation is often used in a way that includes dosage form.
A combination drug or a fixed-dose combination (FDC) is a medicine that includes two or more active ingredients combined in a single dosage form. Terms like "combination drug" or "combination drug product" can be common shorthand for a FDC product, although the latter is more precise if in fact referring to a mass-produced product having a predetermined combination of drugs and respective dosages. And it should also be distinguished from the term "combination product" in medical contexts, which without further specification can refer to products that combine different types of medical products—such as device/drug combinations as opposed to drug/drug combinations. Note that when a combination drug product is a "pill", then it is also a kind of "polypill" or combopill.
Formulation may refer to:

Bromfenac is a nonsteroidal anti-inflammatory drug (NSAID) marketed in the US as an ophthalmic solution by ISTA Pharmaceuticals for short-term, local use. Prolensa and Bromday are the once-daily formulation of bromfenac, while Xibrom was approved for twice-daily administration. In the European Union, the brand name is Yellox. Bromfenac is indicated for the treatment of ocular inflammation and pain after cataract surgery.
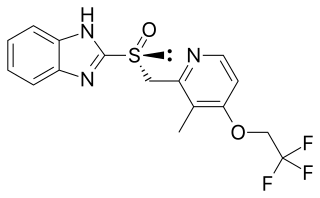
Dexlansoprazole, sold under the trade name Dexilant among others, is a medication which reduces stomach acid. It is used to treat gastroesophageal reflux disease. Effectiveness is similar to other proton pump inhibitors (PPIs). It is taken by mouth.
Formulation is a term used in various senses in various applications, both the material and the abstract or formal. Its fundamental meaning is the putting together of components in appropriate relationships or structures, according to a formula. Etymologically formula is the diminutive of the Latin forma, meaning shape. In that sense a formulation is created according to the standard for the product.

Ipca LaboratoriesLimited is an Indian multinational pharmaceutical company based in Mumbai, India.
The pharmaceutical industry in Pakistan has grown during the past recent decades. At the time of the independence of Pakistan in 1947, there were few production units in the country. Currently Pakistan has more than 800 large volume pharmaceutical formulation units, including those operated by 25 multinationals present in the country. Almost all the raw materials used in making of medicine are sourced from abroad. About 50 percent of them are imported from India.

The National Pharmaceutical Pricing Authority (NPPA) is a government regulatory agency that controls the prices of pharmaceutical drugs in India. National Pharmaceutical Pricing Authority (NPPA) was constituted vide Government of India Resolution dated 29th August 1997 as an attached office of the Department of Pharmaceuticals (DoP), Ministry of Chemicals and Fertilizers as an independent Regulator for pricing of drugs and to ensure availability and accessibility of medicines at affordable prices.

DTaP-IPV vaccine is a combination vaccine whose full generic name is diphtheria and tetanus toxoids and acellular pertussis adsorbed and inactivated poliovirus vaccine (IPV).

Ahmed Hegazy was an Egyptian-German pharmacist. He conducted research in the Pharmaceutical Technology Department of Bayer from 1966 to 1999, during which time he invented a new galenic formulation for the active components nifedipine and nimodipine. By blocking calcium channels, the substance has a relaxing effect on vascular muscles and is therefore used for the treatment of hypertension. In 1991, Hegazy received the Otto Bayer Medal for the solubilization of poorly soluble ingredients such as nimodipine. His galenic invention is still the basis for many modern formulations at Bayer AG.