Gallium selenide may refer to:
| This set index page lists chemical compounds articles associated with the same name. If an internal link led you here, you may wish to change the link to point directly to the intended article. |
Gallium selenide may refer to:
| This set index page lists chemical compounds articles associated with the same name. If an internal link led you here, you may wish to change the link to point directly to the intended article. |
CIGS may refer to:
A selenide is a chemical compound containing a selenium anion with oxidation number of −2 (Se2−), much as sulfur does in a sulfide. The chemistry of the selenides and sulfides is similar. Similar to sulfide, in aqueous solution, the selenide ion, Se2−, is prevalent only in very basic conditions. In neutral conditions, hydrogen selenide ion, HSe−, is most common. In acid conditions, hydrogen selenide, H2Se, is formed.
Chalcogenide glass is a glass containing one or more chalcogens. Such glasses are covalently bonded materials and may be classified as covalent network solids. Polonium is also a chalcogen but is not used because of its strong radioactivity. Chalcogenide materials behave rather differently from oxides, in particular their lower band gaps contribute to very dissimilar optical and electrical properties.
Zinc selenide (ZnSe) is a light-yellow, solid compound comprising zinc (Zn) and selenium (Se). It is an intrinsic semiconductor with a band gap of about 2.70 eV at 25 °C (77 °F). ZnSe rarely occurs in nature, and is found in the mineral that was named after Hans Stille called "stilleite."

Copper indium gallium (di)selenide (CIGS) is a I-III-VI2 semiconductor material composed of copper, indium, gallium, and selenium. The material is a solid solution of copper indium selenide (often abbreviated "CIS") and copper gallium selenide. It has a chemical formula of CuIn(1-x)Ga(x)Se2 where the value of x can vary from 0 (pure copper indium selenide) to 1 (pure copper gallium selenide). CIGS is a tetrahedrally bonded semiconductor, with the chalcopyrite crystal structure, and a bandgap varying continuously with x from about 1.0 eV (for copper indium selenide) to about 1.7 eV (for copper gallium selenide).
Lead selenide (PbSe), or lead(II) selenide, a selenide of lead, is a semiconductor material. It forms cubic crystals of the NaCl structure; it has a direct bandgap of 0.27 eV at room temperature. It is a grey crystalline solid material.
CIG could stand for:
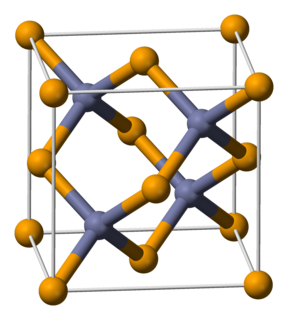
Gallium(III) selenide (Ga2Se3) is a chemical compound. It has a defect sphalerite (cubic form of ZnS) structure. It is a p-type semiconductor
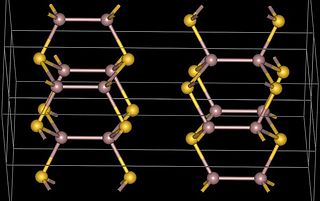
Gallium(II) telluride, GaTe, is a chemical compound of gallium and tellurium. There is research interest in the structure and electronic properties of GaTe because of the possibility that it, or related compounds, may have applications in the electronics industry. Gallium telluride can be made by reacting the elements or by metal organic vapour deposition (MOCVD). . GaTe produced from the elements has a monoclinic crystal structure. Each gallium atom is tetrahedrally coordinated by 3 tellurium and one gallium atom. The gallium-gallium bond length in the Ga2 unit is 2.43 Angstrom. The structure consists of layers and can be formulated as Ga24+ 2Te2−. The bonding within the layers is ionic-covalent and between the layers is predominantly van der Waals. GaTe is classified as a layered semiconductor (like GaSe and InSe which have similar structures). It is a direct band gap semiconductor with an energy of 1.65eV at room temperature. A hexagonal form can be produced by low pressure metal organic vapour deposition (MOCVD) from alkyl gallium telluride cubane-type clusters e.g. from (t-butylGa( μ3-Te))4. The core consists of a cube of eight atoms, four gallium, and four tellurium atoms. Each gallium has an attached t-butyl group and three adjacent tellurium atoms and each tellurium has three adjacent gallium atoms. The hexagonal form, which is closely related to the monoclinic form, containing Ga24+ units, converts to the monoclinic form when annealed at 500 °C.
Tin selenide, also known as stannous selenide, is an inorganic compound with the formula (SnSe), where Tin has a +2 oxidation state. Tin(II) selenide is a narrow band-gap (IV-VI) semiconductor and has received considerable interest for applications including low-cost photovoltaics and memory-switching devices. Tin(II) selenide is a typical layered metal chalcogenide; that is, it includes a Group 16 anion (Se2−) and an electropositive element (Sn2+), and it is arranged in a layered structure.
In chemistry, the Grimm–Sommerfeld rule predicts that binary compounds with covalent character that have an average of 4 electrons per atom will have structures where both atoms are tetrahedrally coordinated. Examples are silicon carbide, the III-V semiconductors indium phosphide and gallium arsenide, the II-VI semiconductors, cadmium sulfide, cadmium selenide.
Indium(III) selenide is a compound of indium and selenium. It has potential for use in photovoltaic devices and it has been the subject of extensive research. The two most common phases, α and β, have a layered structure, while γ is a "defect wurtzite structure." In all, there are five known forms (α, β, γ, δ, κ). The α- β phase transition is accompanied by a change in electrical conductivity. The band-gap of γ-In2Se3 is approximately 1.9 eV. The crystalline form of a sample can depend on the method of production, for example thin films of pure γ-In2Se3 have been produced from trimethylindium, InMe3, and hydrogen selenide, H2Se, using MOCVD techniques.

For the chemical compound, see Gallium(II) selenide. For the American football coach, see Adam Gase. For the American racing driver, see Joey Gase.

Gallium(II) selenide (GaSe) is a chemical compound. It has a hexagonal layer structure, similar to that of GaS. It is a photoconductor, a second harmonic generation crystal in nonlinear optics, and has been used as a far-infrared conversion material at 14-31 THz and above.

A copper indium gallium selenide solar cell is a thin-film solar cell used to convert sunlight into electric power. It is manufactured by depositing a thin layer of copper, indium, gallium and selenium on glass or plastic backing, along with electrodes on the front and back to collect current. Because the material has a high absorption coefficient and strongly absorbs sunlight, a much thinner film is required than of other semiconductor materials.
Solar Frontier Kabushiki Kaisha is a Japanese photovoltaic company that develops and manufactures thin film solar cells using CIGS technology. It is a fully owned subsidiary of Showa Shell Sekiyu and located in Minato, Tokyo, Japan. The company was founded in 2006 as Showa Shell Solar, and renamed Solar Frontier in April 2010.
Copper selenide is an inorganic binary compound consisting of copper and selenium. Its formula is sometimes described as CuSe or Cu2Se, but it is non-stoichiometric. It is a semiconductor and is frequently grown as nanoparticles or other nanostructures.
A triselenide is a compound or ion which contains three selenium atoms or ions. Some examples include: