A selenide is a chemical compound containing a selenium with oxidation number of −2. Similar to sulfide, selenides occur both as inorganic compounds and as organic derivatives, which are called organoselenium compound.

Cadmium selenide is an inorganic compound with the formula CdSe. It is a black to red-black solid that is classified as a II-VI semiconductor of the n-type. It is a pigment, but applications are declining because of environmental concerns.

Zinc selenide is the inorganic compound with the formula ZnSe. It is a lemon-yellow solid although most samples have a duller color due to the effects of oxidation. It is an intrinsic semiconductor with a band gap of about 2.70 eV at 25 °C (77 °F), equivalent to a wavelength of 459 nm. ZnSe occurs as the rare mineral stilleite, named after Hans Stille.

Copper indium gallium (di)selenide (CIGS) is a I-III-VI2 semiconductor material composed of copper, indium, gallium, and selenium. The material is a solid solution of copper indium selenide (often abbreviated "CIS") and copper gallium selenide. It has a chemical formula of CuIn1−xGaxSe2, where the value of x can vary from 0 (pure copper indium selenide) to 1 (pure copper gallium selenide). CIGS is a tetrahedrally bonded semiconductor, with the chalcopyrite crystal structure, and a bandgap varying continuously with x from about 1.0 eV (for copper indium selenide) to about 1.7 eV (for copper gallium selenide).
Lead selenide (PbSe), or lead(II) selenide, a selenide of lead, is a semiconductor material. It forms cubic crystals of the NaCl structure; it has a direct bandgap of 0.27 eV at room temperature. A grey solid, it is used for manufacture of infrared detectors for thermal imaging. The mineral clausthalite is a naturally occurring lead selenide.

Mercury selenide is a chemical compound of mercury and selenium. It is a grey-black crystalline solid semi-metal with a sphalerite structure. The lattice constant is 0.608 nm.
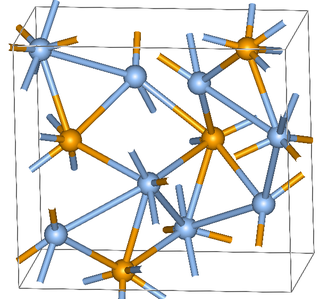
Silver selenide (Ag2Se) is the reaction product formed when selenium toning analog silver gelatine photo papers in photographic print toning. The selenium toner contains sodium selenite (Na2SeO3) as one of its active ingredients, which is the source of the selenide (Se2−) anion combining with the silver in the toning process.

Aluminium selenide is the inorganic compound with the formula Al2Se3.
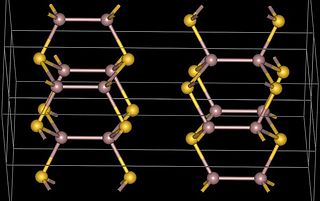
Gallium(II) telluride, GaTe, is a chemical compound of gallium and tellurium. There is research interest in the structure and electronic properties of GaTe because of the possibility that it, or related compounds, may have applications in the electronics industry. Gallium telluride can be made by reacting the elements or by metal organic vapour deposition (MOCVD).

Tin selenide, also known as stannous selenide, is an inorganic compound with the formula SnSe. Tin(II) selenide is a typical layered metal chalcogenide as it includes a group 16 anion (Se2−) and an electropositive element (Sn2+), and is arranged in a layered structure. Tin(II) selenide is a narrow band-gap (IV-VI) semiconductor structurally analogous to black phosphorus. It has received considerable interest for applications including low-cost photovoltaics, and memory-switching devices.
Indium(III) selenide is a compound of indium and selenium. It has potential for use in photovoltaic devices and has been the subject of extensive research. The two most common phases, α and β, have a layered structure, while γ has a "defect wurtzite structure." In all, five polymorphs are known: α, β, γ, δ, κ. The α-β phase transition is accompanied by a change in electrical conductivity. The band gap of γ-In2Se3 is approximately 1.9 eV.

Penroseite is a rare selenide mineral with formula (Ni,Co,Cu)Se2. It has a gray-steel color and black streak with a hardness of 3. It is an isometric mineral, 2/m3. Penroseite was first discovered in 1925 in a Bolivian rhyolite. It was named for Richard Penrose (1863–1931), an economic geologist.

Gallium(II) selenide (GaSe) is a chemical compound. It has a hexagonal layer structure, similar to that of GaS. It is a photoconductor, a second harmonic generation crystal in nonlinear optics, and has been used as a far-infrared conversion material at 14–31 THz and above.

Nickel selenide is the inorganic compound with the formula NiSe. As for many metal chalcogenides, the phase diagram for nickel(II) selenide is complicated. Two other selenides of nickel are known, NiSe2 with a pyrite structure, and Ni2Se3. Additionally, NiSe is usually nonstoichiometric and is often described with the formula Ni1−xSe, with 0 < x < 0.15. This material is a semi-conducting solid, and can be obtained as in the form of a black fine powder, or silver if obtained in the form of larger crystals. Nickel(II) selenide is insoluble in all solvents, but can be degraded by strongly oxidizing acids.
Phosphorus selenides are a relatively obscure group of compounds. There have been some studies of the phosphorus - selenium phase diagram and the glassy amorphous phases are reported. The compounds that have been reported are shown below. While some of phosphorus selenides are similar to their sulfide analogues, there are some new forms, molecular P2Se5 and the polymeric catena-[P4Se4]x. There is also some doubt about the existence of molecular P4Se10.
Copper selenide is an inorganic binary compound between copper and selenium. The chemical formula depends on the ratio between the two elements, such as CuSe or Cu2Se.

Potassium selenide (K2Se) is an inorganic compound formed from selenium and potassium.
Indium(II) selenide (InSe) is an inorganic compound composed of indium and selenium. It is a III-VI layered semiconductor. The solid has a structure consisting of two-dimensional layers bonded together only by van der Waals forces. Each layer has the atoms in the order Se-In-In-Se.
Plutonium selenide is a binary inorganic compound of plutonium and selenium with the chemical formula PuSe. The compound forms black crystals and does not dissolve in water.















