
Carbon monoxide is a colorless, odorless, and tasteless flammable gas that is slightly less dense than air. It is toxic to animals that use hemoglobin as an oxygen carrier when encountered in concentrations above about 35 ppm causing carbon monoxide poisoning. Some carbon monoxide is also produced in normal animal metabolism in low quantities, and is thought to have some normal biological functions. In the atmosphere, it is spatially variable and short-lived, having a role in the formation of ground-level ozone.

Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkaline, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide, cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.

Selenium is a chemical element with the symbol Se and atomic number 34. It is a nonmetal with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, and also has similarities to arsenic. It rarely occurs in its elemental state or as pure ore compounds in the Earth's crust. Selenium—from Ancient Greek σελήνη (selḗnē) "Moon" – was discovered in 1817 by Jöns Jacob Berzelius, who noted the similarity of the new element to the previously discovered tellurium.

Nickel carbonyl (IUPAC name: tetracarbonylnickel) is the organonickel compound with the formula Ni(CO)4. This colorless liquid is the principal carbonyl of nickel. It is an intermediate in the Mond process for producing very high-purity nickel and a reagent in organometallic chemistry, although the Mond Process has fallen out of common usage due to the health hazards in working with the compound. Nickel carbonyl is one of the most dangerous substances yet encountered in nickel chemistry due to its very high toxicity, compounded with high volatility and rapid skin absorption.
A selenide is a chemical compound containing a selenium anion with oxidation number of −2 (Se2−), much as sulfur does in a sulfide. The chemistry of the selenides and sulfides is similar. Similar to sulfide, in aqueous solution, the selenide ion, Se2−, is prevalent only in very basic conditions. In neutral conditions, hydrogen selenide ion, HSe−, is most common. In acid conditions, hydrogen selenide, H2Se, is formed.
Phosphorus trifluoride (formula PF3), is a colorless and odorless gas. It is highly toxic and reacts slowly with water. Its main use is as a ligand in metal complexes. As a ligand, it parallels carbon monoxide in metal carbonyls, and indeed its toxicity is due to its binding with the iron in blood hemoglobin in a similar way to carbon monoxide.
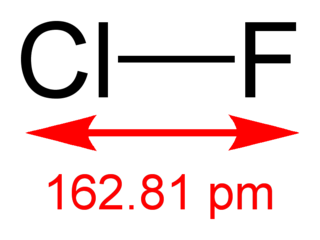
Chlorine monofluoride is a volatile interhalogen compound with the chemical formula ClF. It is a colourless gas at room temperature and is stable even at high temperatures. When cooled to −100 °C, ClF condenses as a pale yellow liquid. Many of its properties are intermediate between its parent halogens, Cl2 and F2.
Hydrogen selenide is an inorganic compound with the formula H2Se. This hydrogen chalcogenide is the simplest and most commonly encountered hydride of selenium. H2Se is a colorless, flammable gas under standard conditions. It is the most toxic selenium compound with an exposure limit of 0.05 ppm over an 8-hour period. Even at extremely low concentrations, this compound has a very irritating smell resembling that of decayed horseradish or 'leaking gas', but smells of rotten eggs at higher concentrations.

Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula Fe(CO)5. Under standard conditions Fe(CO)5 is a free-flowing, straw-colored liquid with a pungent odour. Older samples appear darker. This compound is a common precursor to diverse iron compounds, including many that are useful in small scale organic synthesis.
Organoselenium compounds are chemical compounds containing carbon-to-selenium chemical bonds. Organoselenium chemistry is the corresponding science exploring their properties and reactivity. Selenium belongs with oxygen and sulfur to the group 16 elements or chalcogens, and similarities in chemistry are to be expected. Organoselenium compounds are found at trace levels in ambient waters, soils and sediments.

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes.

The Mond process, sometimes known as the carbonyl process, is a technique created by Ludwig Mond in 1890, to extract and purify nickel. The process was used commercially before the end of the 19th century. This process converts nickel oxides into nickel metal with very high purity being attainable in just a single process.

Dicarbon monoxide (C2O) is a molecule that contains two carbon atoms and one oxygen atom. It is a linear molecule that, because of its simplicity, is of interest in a variety of areas. It is, however, so extremely reactive that it is not encountered in everyday life. It is classified as a cumulene and an oxocarbon.

Dicobalt octacarbonyl is the organometallic compound with composition Co2(CO)8. This metal carbonyl is used as a reagent and catalyst in organometallic chemistry and organic synthesis, and is central to much known organocobalt chemistry. It is the precursor to a hydroformylation catalyst, cobalt tetracarbonyl hydride. Each molecule consists of two cobalt atoms bound to eight carbon monoxide ligands, though multiple distinct structural arrangements are known. Some of the carbonyl ligands are highly labile. The compound is highly reactive towards alkynes, and is sometimes used as an alkyne protecting group. As the cobalt-alkyne complex, it plays a role in promoting both the Nicholas reaction and the Pauson–Khand reaction.
Selenoxide elimination is a method for the chemical synthesis of alkenes from selenoxides. It is most commonly used to synthesize α,β-unsaturated carbonyl compounds from the corresponding saturated analogues. It is mechanistically related to the Cope reaction.

Carbon diselenide is an inorganic compound with the chemical formula CSe2. It is a yellow-orange oily liquid with pungent odor. It is the selenium analogue of carbon disulfide (CS2). This light-sensitive compound is insoluble in water and soluble in organic solvents.

Woollins' reagent is an organic compound containing phosphorus and selenium. Analogous to Lawesson's reagent, it is used mainly as a selenation reagent. It is named after Professor John Derek Woollins.

Selenium monochloride is an inorganic compound with the formula Se2Cl2. Although it is called selenium monochloride, a more descriptive name might be diselenium dichloride. It is a reddish-brown, oily liquid that hydrolyses slowly. It exists in chemical equilibrium with SeCl2, SeCl4, chlorine, and elemental selenium. Selenium monochloride is mainly used as a reagent for the synthesis of Se-containing compounds.

Trifluoromethyl hypofluorite is an organofluorine compound with the formula CF
3OF. It exists as a colorless gas at room temperature and is highly toxic. It is a rare example of a hypofluorite (compound with an O−F bond). It is prepared by the reaction of fluorine gas with carbon monoxide:

Borane carbonyl is the inorganic compound with the formula H3BCO. This colorless gas is the adduct of borane and carbon monoxide. It is usually prepared by combining borane-ether complexes and CO. The compound is mainly of theoretical and pedagogical interest. It reacts with aqueous base to give boranocarbonate H3BCO22−. Bond distances are B−C, 1.529; C−O, 1.140; 1.194 Å. The H−B−H angle is 113.7°. The CO vibrational band is at 2165 cm−1, 22 cm−1 higher than that of free CO.















