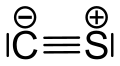| |||
| Names | |||
|---|---|---|---|
| IUPAC name carbon monosulfide | |||
| Other names carbon(II) sulfide, thiocarbonyl, sulfidocarbon, methanidylidynesulfanium | |||
| Identifiers | |||
3D model (JSmol) | |||
| 1697516, 1918616 | |||
| ChEBI | |||
| ChemSpider | |||
| 648 | |||
PubChem CID | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| CS | |||
| Molar mass | 44.07 g·mol−1 | ||
| Appearance | reddish crystalline powder | ||
| insoluble | |||
| Related compounds | |||
Other anions | Carbon monoxide | ||
Other cations | Silicon monosulfide Germanium monosulfide Tin(II) sulfide Lead(II) sulfide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Carbon monosulfide is a chemical compound with the formula CS. This diatomic molecule is the sulfur analogue of carbon monoxide, and is unstable as a solid or a liquid, but it has been observed as a gas both in the laboratory and in the interstellar medium. [1] The molecule resembles carbon monoxide with a triple bond between carbon and sulfur. The molecule is not intrinsically unstable, but it tends to polymerize in sunlight to a brown mass, as first discovered in 1868 and 1872. [2] The polymer is quite stable, decomposing a little at 360 °C to carbon disulfide. This tendency towards polymerization reflects the greater stability of C–S single bonds.
Polymers with the formula (CS)n have been reported, [3] and the formal dimer is ethenedithione. Also, CS has been observed as a ligand in some transition metal thiocarbonyl complexes such as Fe(CO)4CS. [4]
The simplest carbon monosulfide synthesis decomposes carbon disulfide in a high-voltage AC arc. [5]