
Carbon is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust. Three isotopes occur naturally, 12C and 13C being stable, while 14C is a radionuclide, decaying with a half-life of about 5,730 years. Carbon is one of the few elements known since antiquity.

A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ellipsoid, tube, or many other shapes and sizes. Graphene, which is a flat mesh of regular hexagonal rings, can be seen as an extreme member of the family.

Sir Harold Walter Kroto, known as Harry Kroto, was an English chemist. He shared the 1996 Nobel Prize in Chemistry with Robert Curl and Richard Smalley for their discovery of fullerenes. He was the recipient of many other honors and awards.

Richard Errett Smalley was an American chemist who was the Gene and Norman Hackerman Professor of Chemistry, Physics, and Astronomy at Rice University. In 1996, along with Robert Curl, also a professor of chemistry at Rice, and Harold Kroto, a professor at the University of Sussex, he was awarded the Nobel Prize in Chemistry for the discovery of a new form of carbon, buckminsterfullerene, also known as buckyballs. He was an advocate of nanotechnology and its applications.

Robert Floyd Curl Jr. was an American chemist who was Pitzer–Schlumberger Professor of Natural Sciences and Professor of Chemistry at Rice University. He was awarded the Nobel Prize in Chemistry in 1996 for the discovery of the nanomaterial buckminsterfullerene, and hence the fullerene class of materials, along with Richard Smalley and Harold Kroto of the University of Sussex.

In geometry, the truncated icosahedron is an Archimedean solid, one of 13 convex isogonal nonprismatic solids whose 32 faces are two or more types of regular polygons. It is the only one of these shapes that does not contain triangles or squares. In general usage, the degree of truncation is assumed to be uniform unless specified.

Buckminsterfullerene is a type of fullerene with the formula C60. It has a cage-like fused-ring structure (truncated icosahedron) made of twenty hexagons and twelve pentagons, and resembles a soccer ball. Each of its 60 carbon atoms is bonded to its three neighbors.
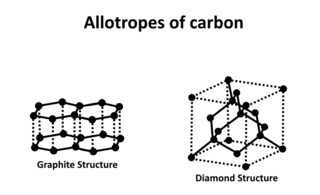
Carbon is capable of forming many allotropes due to its valency. Well-known forms of carbon include diamond and graphite. In recent decades, many more allotropes have been discovered and researched, including ball shapes such as buckminsterfullerene and sheets such as graphene. Larger-scale structures of carbon include nanotubes, nanobuds and nanoribbons. Other unusual forms of carbon exist at very high temperatures or extreme pressures. Around 500 hypothetical 3‑periodic allotropes of carbon are known at the present time, according to the Samara Carbon Allotrope Database (SACADA).
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.

Fullerene chemistry is a field of organic chemistry devoted to the chemical properties of fullerenes. Research in this field is driven by the need to functionalize fullerenes and tune their properties. For example, fullerene is notoriously insoluble and adding a suitable group can enhance solubility. By adding a polymerizable group, a fullerene polymer can be obtained. Functionalized fullerenes are divided into two classes: exohedral fullerenes with substituents outside the cage and endohedral fullerenes with trapped molecules inside the cage.
The history of nanotechnology traces the development of the concepts and experimental work falling under the broad category of nanotechnology. Although nanotechnology is a relatively recent development in scientific research, the development of its central concepts happened over a longer period of time. The emergence of nanotechnology in the 1980s was caused by the convergence of experimental advances such as the invention of the scanning tunneling microscope in 1981 and the discovery of fullerenes in 1985, with the elucidation and popularization of a conceptual framework for the goals of nanotechnology beginning with the 1986 publication of the book Engines of Creation. The field was subject to growing public awareness and controversy in the early 2000s, with prominent debates about both its potential implications as well as the feasibility of the applications envisioned by advocates of molecular nanotechnology, and with governments moving to promote and fund research into nanotechnology. The early 2000s also saw the beginnings of commercial applications of nanotechnology, although these were limited to bulk applications of nanomaterials rather than the transformative applications envisioned by the field.
In materials science, an interstitial defect is a type of point crystallographic defect where an atom of the same or of a different type, occupies an interstitial site in the crystal structure. When the atom is of the same type as those already present they are known as a self-interstitial defect. Alternatively, small atoms in some crystals may occupy interstitial sites, such as hydrogen in palladium. Interstitials can be produced by bombarding a crystal with elementary particles having energy above the displacement threshold for that crystal, but they may also exist in small concentrations in thermodynamic equilibrium. The presence of interstitial defects can modify the physical and chemical properties of a material.
James R. Heath is an American chemist and the president and professor of Institute of Systems Biology. Previous to this, he was the Elizabeth W. Gilloon Professor of Chemistry at the California Institute of Technology, after having moved from University of California Los Angeles.

Linear acetylenic carbon (LAC), also known as carbyne or Linear Carbon Chain (LCC), is an allotrope of carbon that has the chemical structure (−C≡C−)n as a repeat unit, with alternating single and triple bonds. It would thus be the ultimate member of the polyyne family.
A buckyball or buckminsterfullerene is a molecule resembling a soccer ball composed of 60 carbon atoms.
Azafullerenes are a class of heterofullerenes in which the element substituting for carbon is nitrogen. They can be in the form of a hollow sphere, ellipsoid, tube, and many other shapes. Spherical azafullerenes resemble the balls used in football (soccer). They are also a member of the carbon nitride class of materials that include beta carbon nitride (β-C3N4), predicted to be harder than diamond. Besides the pioneering work of a couple of academic groups, this class of compounds has so far garnered little attention from the broader fullerene research community. Many properties and structures are yet to be discovered for the highly-nitrogen substituted subset of molecules.
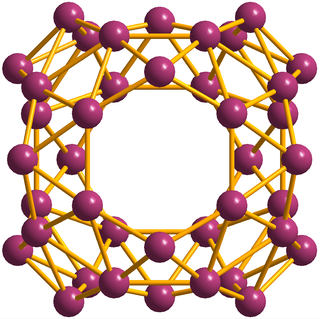
Borospherene (B40) is a cluster molecule containing 40 boron atoms. It is similar to buckminsterfullerene, the "spherical" carbon structure, but with a different symmetry. The discovery of borospherene was announced in July 2014, and is described in the journal Nature Chemistry. Borospherene is similar to other cluster molecules, including buckminsterfullerene (C60), stannaspherene, and plumbaspherene. The molecule includes unusual heptagonal faces.

Nanoclusters are atomically precise, crystalline materials most often existing on the 0-2 nanometer scale. They are often considered kinetically stable intermediates that form during the synthesis of comparatively larger materials such as semiconductor and metallic nanocrystals. The majority of research conducted to study nanoclusters has focused on characterizing their crystal structures and understanding their role in the nucleation and growth mechanisms of larger materials. These nanoclusters can be composed either of a single or of multiple elements, and exhibit interesting electronic, optical, and chemical properties compared to their larger counterparts.

Konstantinos Fostiropoulos is a Greek physicist who has been working in Germany in the areas nano-materials, solid-state physics, molecular physics, astrophysics, and thermodynamics. From 2003 to 2016 he has been founder and head of the Organic Solar Cells Group at the Institute Heterogeneous Materials Systems within the Helmholtz-Zentrum Berlin. His scientific works include novel energy materials and photovoltaic device concepts, carbon clusters in the Interstellar Medium, and intermolecular forces of real gases.

The solubility of fullerenes is generally low. Carbon disulfide dissolves 8g/L of C60, and the best solvent (1-chloronaphthalene) dissolves 53 g/L. up Still, fullerenes are the only known allotrope of carbon that can be dissolved in common solvents at room temperature. Besides those two, good solvents for fullerenes include 1,2-dichlorobenzene, toluene, p-xylene, and 1,2,3-tribromopropane. Fullerenes are highly insoluble in water, and practically insoluble in methanol.



















