| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Formamide [1] | |||
| Systematic IUPAC name Methanamide | |||
| Other names Carbamaldehyde | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.766 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID | |||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
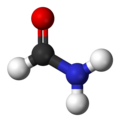
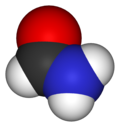
| CH3NO | |||
| Molar mass | 45.04 g/mol | ||
| Appearance | Colorless, oily liquid [2] | ||
| Density | 1.133 g/cm3 | ||
| Melting point | 2 to 3 °C (36 to 37 °F; 275 to 276 K) | ||
| Boiling point | 210 °C (410 °F; 483 K) | ||
| Miscible | |||
| Vapor pressure | 0.08 mmHg at 20 °C | ||
| Acidity (pKa) | 23.5 (in DMSO) [3] | ||
| −2.19×10−5 cm3/mol | |||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 154 °C (309 °F; 427 K) (closed cup) | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) | none [2] | ||
REL (Recommended) | TWA 10 ppm (15 mg/m3) [skin] [2] | ||
IDLH (Immediate danger) | N.D. [2] | ||
| Related compounds | |||
Related compounds | Carbamic acid Dimethylformamide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Formamide is an amide derived from formic acid. It is a colorless liquid which is miscible with water and has an ammonia-like odor. It is chemical feedstock for the manufacture of sulfa drugs and other pharmaceuticals, herbicides and pesticides, and in the manufacture of hydrocyanic acid. It has been used as a softener for paper and fiber. It is a solvent for many ionic compounds. It has also been used as a solvent for resins and plasticizers. [4] Some astrobiologists suggest that it may be an alternative to water as the main solvent in other forms of life. [5]
Contents
- Production
- Historical production
- Modern production
- Applications
- Reactions
- Niche or laboratory applications
- Biochemistry
- Prebiotic chemistry
- Safety
- References
Formamides are compounds of the type RR′NCHO. One important formamide is dimethylformamide, (CH3)2NCHO.