
A molecular cloud, sometimes called a stellar nursery (if star formation is occurring within), is a type of interstellar cloud, the density and size of which permit absorption nebulae, the formation of molecules (most commonly molecular hydrogen, H2), and the formation of H II regions. This is in contrast to other areas of the interstellar medium that contain predominantly ionized gas.

In astronomy, the interstellar medium (ISM) is the matter and radiation that exist in the space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as dust and cosmic rays. It fills interstellar space and blends smoothly into the surrounding intergalactic space. The energy that occupies the same volume, in the form of electromagnetic radiation, is the interstellar radiation field. Although the density of atoms in the ISM is usually far below that in the best laboratory vacuums, the mean free path between collisions is short compared to typical interstellar lengths, so on these scales the ISM behaves as a gas (more precisely, as a plasma: it is everywhere at least slightly ionized), responding to pressure forces, and not as a collection of non-interacting particles.

Astrochemistry is the study of the abundance and reactions of molecules in the universe, and their interaction with radiation. The discipline is an overlap of astronomy and chemistry. The word "astrochemistry" may be applied to both the Solar System and the interstellar medium. The study of the abundance of elements and isotope ratios in Solar System objects, such as meteorites, is also called cosmochemistry, while the study of interstellar atoms and molecules and their interaction with radiation is sometimes called molecular astrophysics. The formation, atomic and chemical composition, evolution and fate of molecular gas clouds is of special interest, because it is from these clouds that solar systems form.

The hydroxyl radical is the diatomic molecule •
OH. The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals are produced from the decomposition of hydroperoxides (ROOH) or, in atmospheric chemistry, by the reaction of excited atomic oxygen with water. It is also important in the field of radiation chemistry, since it leads to the formation of hydrogen peroxide and oxygen, which can enhance corrosion and SCC in coolant systems subjected to radioactive environments. The unpaired electron of the hydroxyl radical is officially represented by a middle dot, •, beside the O.

The trihydrogen cation or protonated molecular hydrogen is a cation with formula H+
3, consisting of three hydrogen nuclei (protons) sharing two electrons.

CW Leonis or IRC +10216 is a variable carbon star that is embedded in a thick dust envelope. It was first discovered in 1969 by a group of astronomers led by Eric Becklin, based upon infrared observations made with the 62 inches (1.6 m) Caltech Infrared Telescope at Mount Wilson Observatory. Its energy is emitted mostly at infrared wavelengths. At a wavelength of 5 μm, it was found to have the highest flux of any object outside the Solar System.
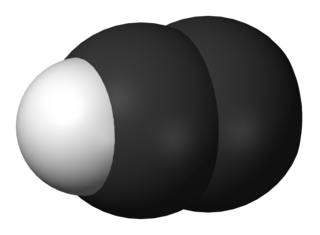
The ethynyl radical (systematically named λ3-ethyne and hydridodicarbon(C—C)) is an organic compound with the chemical formula C≡CH (also written [CCH] or C
2H). It is a simple molecule that does not occur naturally on Earth but is abundant in the interstellar medium. It was first observed by electron spin resonance isolated in a solid argon matrix at liquid helium temperatures in 1963 by Cochran and coworkers at the Johns Hopkins Applied Physics Laboratory. It was first observed in the gas phase by Tucker and coworkers in November 1973 toward the Orion Nebula, using the NRAO 11-meter radio telescope. It has since been detected in a large variety of interstellar environments, including dense molecular clouds, bok globules, star forming regions, the shells around carbon-rich evolved stars, and even in other galaxies.
Hydrogen isocyanide is a chemical with the molecular formula HNC. It is a minor tautomer of hydrogen cyanide (HCN). Its importance in the field of astrochemistry is linked to its ubiquity in the interstellar medium.
Octatetraynyl radical is an organic free radical with eight carbon atoms linked in a linear chain with alternating single bonds and triple bonds.

HCNH+, also known as protonated hydrogen cyanide, is a molecular ion of astrophysical interest. It also exists in the condensed state when formed by superacids.

Cyclopropenylidene, or c-C3H2, is a partially aromatic molecule belonging to a highly reactive class of organic molecules known as carbenes. On Earth, cyclopropenylidene is only seen in the laboratory due to its reactivity. However, cyclopropenylidene is found in significant concentrations in the interstellar medium (ISM) and on Saturn's moon Titan. Its C2v symmetric isomer, propadienylidene (CCCH2) is also found in the ISM, but with abundances about an order of magnitude lower. A third C2 symmetric isomer, propargylene (HCCCH), has not yet been detected in the ISM, most likely due to its low dipole moment.

The cyano radical (or cyanido radical) is a radical with molecular formula CN, sometimes written •CN. The cyano radical was one of the first detected molecules in the interstellar medium, in 1938. Its detection and analysis was influential in astrochemistry. The discovery was confirmed with a coudé spectrograph, which was made famous and credible due to this detection. ·CN has been observed in both diffuse clouds and dense clouds. Usually, CN is detected in regions with hydrogen cyanide, hydrogen isocyanide, and HCNH+, since it is involved in the creation and destruction of these species (see also Cyanogen).
In organic chemistry, cyanopolyynes are a family of organic compounds with the chemical formula HCnN (n = 3,5,7,…) and the structural formula H−[C≡C−]nC≡N (n = 1,2,3,…). Structurally, they are polyynes with a cyano group (−C≡N) covalently bonded to one of the terminal acetylene units (H−C≡C).

Imidogen is an inorganic compound with the chemical formula NH. Like other simple radicals, it is highly reactive and consequently short-lived except as a dilute gas. Its behavior depends on its spin multiplicity.
Stellar molecules are molecules that exist or form in stars. Such formations can take place when the temperature is low enough for molecules to form – typically around 6000 K or cooler. Otherwise the stellar matter is restricted to atoms in the forms of gas or – at very high temperatures – plasma.
A heterocumulene is a molecule or ion containing a chain of at least three double bonds between consecutive atoms, in which one or more atoms in the doubly bonded chain is a heteroatom. Such species are analogous to a cumulene in which the chain of doubly bonded atoms contains only carbon, except that at least one carbon is replaced by a heteroatom. Some authors relax the definition to include species with chains of only two double bonds between consecutive atoms, also known as heteroallenes.
Tricarbon monosulfide (C3S) or tricarbon sulfur is a reactive molecular substance that has been detected in outer space. Tricarbon monosulfide is a heterocumulene or thiocumulene, consisting of a straight chain of three carbon atoms and a terminal sulfur atom.

Phosphorus monoxide is an unstable radical inorganic compound with molecular formula PO.

HD 73882 is a visual binary system with the components separated by 0.6″ and a combined spectral class of O8. One of stars is an eclipsing binary system. The period of variability is listed as both 2.9199 days and 20.6 days, possibly due to the secondary being a spectroscopic binary star.













