Triatomic molecules are molecules composed of three atoms, of either the same or different chemical elements. Examples include H2O, CO2 (pictured), HCN, O3 (ozone) and NO2.

Triatomic molecules are molecules composed of three atoms, of either the same or different chemical elements. Examples include H2O, CO2 (pictured), HCN, O3 (ozone) and NO2.
The vibrational modes of a triatomic molecule can be determined in specific cases.
A symmetric linear molecule ABA can perform:
In the previous formulas, M is the total mass of the molecule, mA and mB are the masses of the elements A and B, k1 and k2 are the spring constants of the molecule along its axis and perpendicular to it.


Homonuclear triatomic molecules contain three of the same kind of atom. That molecule will be an allotrope of that element.
Ozone, O3 is an example of a triatomic molecule with all atoms the same. Triatomic hydrogen, H3, is unstable and breaks up spontaneously. H3+, the trihydrogen cation is stable by itself and is symmetric. 4He3, the helium trimer is only weakly bound by van der Waals force and is in an Efimov state. [1] Trisulfur (S3) is analogous to ozone.
All triatomic molecules may be classified as possessing either a linear, bent, or cyclic geometry.[ further explanation needed ]
Linear triatomic molecules owe their geometry to their sp or sp3d hybridised central atoms. Well-known linear triatomic molecules include carbon dioxide (CO2) and hydrogen cyanide (HCN).
Xenon difluoride (XeF2) is one of the rare examples of a linear triatomic molecule possessing non-bonded pairs of electrons on the central atom.

In physics, a dipole is an electromagnetic phenomenon which occurs in two ways:

Oscillation is the repetitive or periodic variation, typically in time, of some measure about a central value or between two or more different states. Familiar examples of oscillation include a swinging pendulum and alternating current. Oscillations can be used in physics to approximate complex interactions, such as those between atoms.

In physics and electrical engineering, a cutoff frequency, corner frequency, or break frequency is a boundary in a system's frequency response at which energy flowing through the system begins to be reduced rather than passing through.
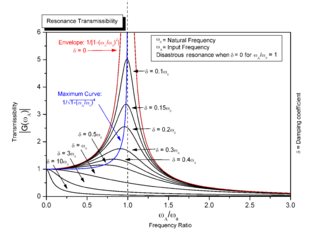
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force is equal or close to a natural frequency of the system on which it acts. When an oscillating force is applied at a resonant frequency of a dynamic system, the system will oscillate at a higher amplitude than when the same force is applied at other, non-resonant frequencies.

The quantum harmonic oscillator is the quantum-mechanical analog of the classical harmonic oscillator. Because an arbitrary smooth potential can usually be approximated as a harmonic potential at the vicinity of a stable equilibrium point, it is one of the most important model systems in quantum mechanics. Furthermore, it is one of the few quantum-mechanical systems for which an exact, analytical solution is known.

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion. Ionization can result from the loss of an electron after collisions with subatomic particles, collisions with other atoms, molecules and ions, or through the interaction with electromagnetic radiation. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.

In physics, a phonon is a collective excitation in a periodic, elastic arrangement of atoms or molecules in condensed matter, specifically in solids and some liquids. A type of quasiparticle, a phonon is an excited state in the quantum mechanical quantization of the modes of vibrations for elastic structures of interacting particles. Phonons can be thought of as quantized sound waves, similar to photons as quantized light waves.

In thermodynamics and solid-state physics, the Debye model is a method developed by Peter Debye in 1912 for estimating the phonon contribution to the specific heat in a solid. It treats the vibrations of the atomic lattice (heat) as phonons in a box, in contrast to the Einstein photoelectron model, which treats the solid as many individual, non-interacting quantum harmonic oscillators. The Debye model correctly predicts the low-temperature dependence of the heat capacity of solids, which is proportional to – the Debye T 3 law. Similarly to the Einstein photoelectron model, it recovers the Dulong–Petit law at high temperatures. Due to simplifying assumptions, its accuracy suffers at intermediate temperatures.

A normal mode of a dynamical system is a pattern of motion in which all parts of the system move sinusoidally with the same frequency and with a fixed phase relation. The free motion described by the normal modes takes place at fixed frequencies. These fixed frequencies of the normal modes of a system are known as its natural frequencies or resonant frequencies. A physical object, such as a building, bridge, or molecule, has a set of normal modes and their natural frequencies that depend on its structure, materials and boundary conditions.
Rotational–vibrational spectroscopy is a branch of molecular spectroscopy concerned with infrared and Raman spectra of molecules in the gas phase. Transitions involving changes in both vibrational and rotational states can be abbreviated as rovibrational transitions. When such transitions emit or absorb photons, the frequency is proportional to the difference in energy levels and can be detected by certain kinds of spectroscopy. Since changes in rotational energy levels are typically much smaller than changes in vibrational energy levels, changes in rotational state are said to give fine structure to the vibrational spectrum. For a given vibrational transition, the same theoretical treatment as for pure rotational spectroscopy gives the rotational quantum numbers, energy levels, and selection rules. In linear and spherical top molecules, rotational lines are found as simple progressions at both higher and lower frequencies relative to the pure vibration frequency. In symmetric top molecules the transitions are classified as parallel when the dipole moment change is parallel to the principal axis of rotation, and perpendicular when the change is perpendicular to that axis. The ro-vibrational spectrum of the asymmetric rotor water is important because of the presence of water vapor in the atmosphere.

Rotational spectroscopy is concerned with the measurement of the energies of transitions between quantized rotational states of molecules in the gas phase. The spectra of polar molecules can be measured in absorption or emission by microwave spectroscopy or by far infrared spectroscopy. The rotational spectra of non-polar molecules cannot be observed by those methods, but can be observed and measured by Raman spectroscopy. Rotational spectroscopy is sometimes referred to as pure rotational spectroscopy to distinguish it from rotational-vibrational spectroscopy where changes in rotational energy occur together with changes in vibrational energy, and also from ro-vibronic spectroscopy where rotational, vibrational and electronic energy changes occur simultaneously.
In rotordynamics, the rigid rotor is a mechanical model of rotating systems. An arbitrary rigid rotor is a 3-dimensional rigid object, such as a top. To orient such an object in space requires three angles, known as Euler angles. A special rigid rotor is the linear rotor requiring only two angles to describe, for example of a diatomic molecule. More general molecules are 3-dimensional, such as water, ammonia, or methane.

In classical mechanics, anharmonicity is the deviation of a system from being a harmonic oscillator. An oscillator that is not oscillating in harmonic motion is known as an anharmonic oscillator where the system can be approximated to a harmonic oscillator and the anharmonicity can be calculated using perturbation theory. If the anharmonicity is large, then other numerical techniques have to be used. In reality all oscillating systems are anharmonic, but most approximate the harmonic oscillator the smaller the amplitude of the oscillation is.
The vibrational partition function traditionally refers to the component of the canonical partition function resulting from the vibrational degrees of freedom of a system. The vibrational partition function is only well-defined in model systems where the vibrational motion is relatively uncoupled with the system's other degrees of freedom.
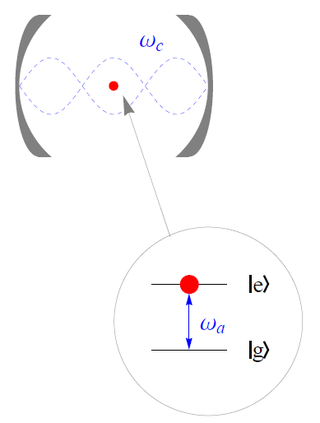
The Jaynes–Cummings model is a theoretical model in quantum optics. It describes the system of a two-level atom interacting with a quantized mode of an optical cavity, with or without the presence of light. It was originally developed to study the interaction of atoms with the quantized electromagnetic field in order to investigate the phenomena of spontaneous emission and absorption of photons in a cavity.
A molecular vibration is a periodic motion of the atoms of a molecule relative to each other, such that the center of mass of the molecule remains unchanged. The typical vibrational frequencies range from less than 1013 Hz to approximately 1014 Hz, corresponding to wavenumbers of approximately 300 to 3000 cm−1 and wavelengths of approximately 30 to 3 µm.

In solid state physics, a surface phonon is the quantum of a lattice vibration mode associated with a solid surface. Similar to the ordinary lattice vibrations in a bulk solid, the nature of surface vibrations depends on details of periodicity and symmetry of a crystal structure. Surface vibrations are however distinct from the bulk vibrations, as they arise from the abrupt termination of a crystal structure at the surface of a solid. Knowledge of surface phonon dispersion gives important information related to the amount of surface relaxation, the existence and distance between an adsorbate and the surface, and information regarding presence, quantity, and type of defects existing on the surface.
Triatomic hydrogen or H3 is an unstable triatomic molecule containing only hydrogen. Since this molecule contains only three atoms of hydrogen it is the simplest triatomic molecule and it is relatively simple to numerically solve the quantum mechanics description of the particles. Being unstable the molecule breaks up in under a millionth of a second. Its fleeting lifetime makes it rare, but it is quite commonly formed and destroyed in the universe thanks to the commonness of the trihydrogen cation. The infrared spectrum of H3 due to vibration and rotation is very similar to that of the ion, H+
3. In the early universe this ability to emit infrared light allowed the primordial hydrogen and helium gas to cool down so as to form stars.

In condensed matter physics, the Lyddane–Sachs–Teller relation determines the ratio of the natural frequency of longitudinal optic lattice vibrations (phonons) of an ionic crystal to the natural frequency of the transverse optical lattice vibration for long wavelengths. The ratio is that of the static permittivity to the permittivity for frequencies in the visible range .

The helium trimer is a weakly bound molecule consisting of three helium atoms. Van der Waals forces link the atoms together. The combination of three atoms is much more stable than the two-atom helium dimer. The three-atom combination of helium-4 atoms is an Efimov state. Helium-3 is predicted to form a trimer, although ground state dimers containing helium-3 are completely unstable.