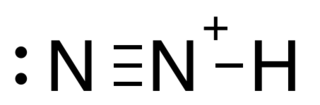A molecular cloud, sometimes called a stellar nursery (if star formation is occurring within), is a type of interstellar cloud, the density and size of which permit absorption nebulae, the formation of molecules (most commonly molecular hydrogen, H2), and the formation of H II regions. This is in contrast to other areas of the interstellar medium that contain predominantly ionized gas.
Hydrogen cyanide is a chemical compound with the formula HCN and structural formula H−C≡N. It is a highly toxic and flammable liquid that boils slightly above room temperature, at 25.6 °C (78.1 °F). HCN is produced on an industrial scale and is a highly valued precursor to many chemical compounds ranging from polymers to pharmaceuticals. Large-scale applications are for the production of potassium cyanide and adiponitrile, used in mining and plastics, respectively. It is more toxic than solid cyanide compounds due to its volatile nature. A solution of hydrogen cyanide in water, represented as HCN, is called hydrocyanic acid. The salts of the cyanide anion are known as cyanides.
In chemistry, hydronium is the cation [H3O]+, also written as H3O+, the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid is dissolved in water, as Arrhenius acid molecules in solution give up a proton to the surrounding water molecules. In fact, acids must be surrounded by more than a single water molecule in order to ionize, yielding aqueous H+ and conjugate base.

Astrochemistry is the study of the abundance and reactions of molecules in the universe, and their interaction with radiation. The discipline is an overlap of astronomy and chemistry. The word "astrochemistry" may be applied to both the Solar System and the interstellar medium. The study of the abundance of elements and isotope ratios in Solar System objects, such as meteorites, is also called cosmochemistry, while the study of interstellar atoms and molecules and their interaction with radiation is sometimes called molecular astrophysics. The formation, atomic and chemical composition, evolution and fate of molecular gas clouds is of special interest, because it is from these clouds that solar systems form.
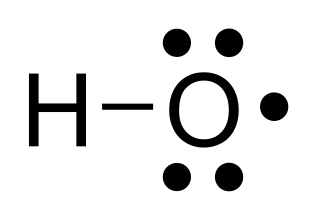
The hydroxyl radical, •HO, is the neutral form of the hydroxide ion (HO–). Hydroxyl radicals are highly reactive and consequently short-lived; however, they form an important part of radical chemistry. Most notably hydroxyl radicals are produced from the decomposition of hydroperoxides (ROOH) or, in atmospheric chemistry, by the reaction of excited atomic oxygen with water. It is also an important radical formed in radiation chemistry, since it leads to the formation of hydrogen peroxide and oxygen, which can enhance corrosion and stress corrosion cracking in coolant systems subjected to radioactive environments. Hydroxyl radicals are also produced during UV-light dissociation of H2O2 (suggested in 1879) and likely in Fenton chemistry, where trace amounts of reduced transition metals catalyze peroxide-mediated oxidations of organic compounds.

The trihydrogen cation or protonated molecular hydrogen is a cation with formula H+3, consisting of three hydrogen nuclei (protons) sharing two electrons.
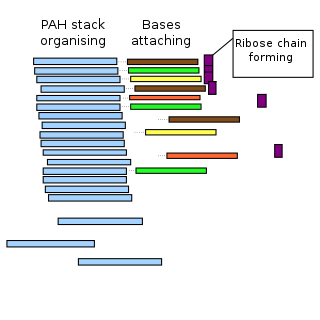
The PAH world hypothesis is a speculative hypothesis that proposes that polycyclic aromatic hydrocarbons (PAHs), known to be abundant in the universe, including in comets, and assumed to be abundant in the primordial soup of the early Earth, played a major role in the origin of life by mediating the synthesis of RNA molecules, leading into the RNA world. However, as yet, the hypothesis is untested.
The ethynyl radical (systematically named λ3-ethyne and hydridodicarbon(C—C)) is an organic compound with the chemical formula C≡CH (also written [CCH] or C
2H). It is a simple molecule that does not occur naturally on Earth but is abundant in the interstellar medium. It was first observed by electron spin resonance isolated in a solid argon matrix at liquid helium temperatures in 1963 by Cochran and coworkers at the Johns Hopkins Applied Physics Laboratory. It was first observed in the gas phase by Tucker and coworkers in November 1973 toward the Orion Nebula, using the NRAO 11-meter radio telescope. It has since been detected in a large variety of interstellar environments, including dense molecular clouds, bok globules, star forming regions, the shells around carbon-rich evolved stars, and even in other galaxies.
Sagittarius B2 is a giant molecular cloud of gas and dust that is located about 120 parsecs (390 ly) from the center of the Milky Way. This complex is the largest molecular cloud in the vicinity of the core and one of the largest in the galaxy, spanning a region about 45 parsecs (150 ly) across. The total mass of Sgr B2 is about 3 million times the mass of the Sun. The mean hydrogen density within the cloud is 3000 atoms per cm3, which is about 20–40 times denser than a typical molecular cloud.
Propynylidyne is a chemical compound that has been identified in interstellar space.
Interstellar formaldehyde (a topic relevant to astrochemistry) was first discovered in 1969 by L. Snyder et al. using the National Radio Astronomy Observatory. Formaldehyde (H2CO) was detected by means of the 111 - 110 ground state rotational transition at 4830 MHz. On 11 August 2014, astronomers released studies, using the Atacama Large Millimeter/Submillimeter Array (ALMA) for the first time, that detailed the distribution of HCN, HNC, H2CO, and dust inside the comae of comets C/2012 F6 (Lemmon) and C/2012 S1 (ISON).
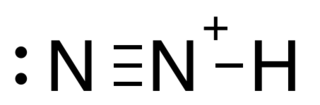
Diazenylium is the chemical N2H+, an inorganic cation that was one of the first ions to be observed in interstellar clouds. Since then, it has been observed for in several different types of interstellar environments, observations that have several different scientific uses. It gives astronomers information about the fractional ionization of gas clouds, the chemistry that happens within those clouds, and it is often used as a tracer for molecules that are not as easily detected (such as N2). Its 1–0 rotational transition occurs at 93.174 GHz, a region of the spectrum where Earth's atmosphere is transparent and it has a significant optical depth in both cold and warm clouds so it is relatively easy to observe with ground-based observatories. The results of N2H+ observations can be used not only for determining the chemistry of interstellar clouds, but also for mapping the density and velocity profiles of these clouds.

HCNH+, also known as protonated hydrogen cyanide, is a molecular ion of astrophysical interest. It also exists in the condensed state when formed by superacids.

Cyclopropenylidene, or c-C3H2, is a partially aromatic molecule belonging to a highly reactive class of organic molecules known as carbenes. On Earth, cyclopropenylidene is only seen in the laboratory due to its reactivity. However, cyclopropenylidene is found in significant concentrations in the interstellar medium (ISM) and on Saturn's moon Titan. Its C2v symmetric isomer, propadienylidene (CCCH2) is also found in the ISM, but with abundances about an order of magnitude lower. A third C2 symmetric isomer, propargylene (HCCCH), has not yet been detected in the ISM, most likely due to its low dipole moment.

The cyano radical (or cyanido radical) is a radical with molecular formula CN, sometimes written •CN. The cyano radical was one of the first detected molecules in the interstellar medium, in 1938. Its detection and analysis was influential in astrochemistry. The discovery was confirmed with a coudé spectrograph, which was made famous and credible due to this detection. ·CN has been observed in both diffuse clouds and dense clouds. Usually, CN is detected in regions with hydrogen cyanide, hydrogen isocyanide, and HCNH+, since it is involved in the creation and destruction of these species (see also Cyanogen).
In organic chemistry, cyanopolyynes are a family of organic compounds with the chemical formula HCnN (n = 3,5,7,…) and the structural formula H−[C≡C−]nC≡N (n = 1,2,3,…). Structurally, they are polyynes with a cyano group (−C≡N) covalently bonded to one of the terminal acetylene units (H−C≡C).

Imidogen is an inorganic compound with the chemical formula NH. Like other simple radicals, it is highly reactive and consequently short-lived except as a dilute gas. Its behavior depends on its spin multiplicity.
Tricarbon monosulfide (C3S) or tricarbon sulfur is a reactive molecular substance that has been detected in outer space. Tricarbon monosulfide is a heterocumulene or thiocumulene, consisting of a straight chain of three carbon atoms and a terminal sulfur atom.
C/2000 WM1 (LINEAR) is a non-periodic comet discovered by LINEAR on 16 December 2000. The comet brightened to an apparent magnitude of about 2.5.