
Perchloric acid is a mineral acid with the formula HClO4. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid, nitric acid and hydrochloric acid. It is a powerful oxidizer when hot, but aqueous solutions up to approximately 70% by weight at room temperature are generally safe, only showing strong acid features and no oxidizing properties. Perchloric acid is useful for preparing perchlorate salts, especially ammonium perchlorate, an important rocket fuel component. Perchloric acid is dangerously corrosive and readily forms potentially explosive mixtures.
Chromic acid is an inorganic acid composed of the elements chromium, oxygen, and hydrogen. It is a dark, purplish red, odorless, sand-like solid powder. When dissolved in water, it is a strong acid. There are 2 types of chromic acid: molecular chromic acid with the formula H
2CrO
4 and dichromic acid with the formula H
2Cr
2O
7.
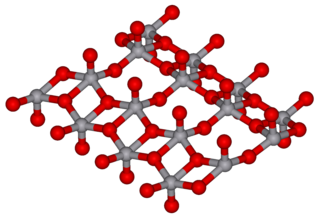
Vanadium(V) oxide (vanadia) is the inorganic compound with the formula V2O5. Commonly known as vanadium pentoxide, it is a brown/yellow solid, although when freshly precipitated from aqueous solution, its colour is deep orange. Because of its high oxidation state, it is both an amphoteric oxide and an oxidizing agent. From the industrial perspective, it is the most important compound of vanadium, being the principal precursor to alloys of vanadium and is a widely used industrial catalyst.

Sodium perchlorate is an inorganic compound with the chemical formula NaClO4. It consists of sodium cations Na+ and perchlorate anions ClO−4. It is a white crystalline, hygroscopic solid that is highly soluble in water and ethanol. It is usually encountered as sodium perchlorate monohydrate NaClO4·H2O. The compound is noteworthy as the most water-soluble of the common perchlorate salts.

Telluric acid, or more accurately orthotelluric acid, is a chemical compound with the formula Te(OH)6, often written as H6TeO6. It is a white crystalline solid made up of octahedral Te(OH)6 molecules which persist in aqueous solution. In the solid state, there are two forms, rhombohedral and monoclinic, and both contain octahedral Te(OH)6 molecules, containing one hexavalent tellurium (Te) atom in the +6 oxidation state, attached to six hydroxyl (–OH) groups, thus, it can be called tellurium(VI) hydroxide. Telluric acid is a weak acid which is dibasic, forming tellurate salts with strong bases and hydrogen tellurate salts with weaker bases or upon hydrolysis of tellurates in water. It is used as tellurium-source in the synthesis of oxidation catalysts.

Selenium dioxide is the chemical compound with the formula SeO2. This colorless solid is one of the most frequently encountered compounds of selenium.

Selenite refers to the anion with the chemical formula SeO2−3. It is the oxyanion of selenium. It is the selenium analog of the sulfite ion, SO2−3. Thus selenite is pyramidal and selenium is assigned oxidation state +4. Selenite also refers to compounds that contains this ion, for example sodium selenite Na2SeO3 which is a common source of selenite. Selenite also refers to the esters of selenous acid, for example dimethyl selenite (CH3)2SeO3.

Selenous acid is the chemical compound with the formula H2SeO3. Structurally, it is more accurately described by O=Se(OH)2. It is the principal oxoacid of selenium; the other being selenic acid.
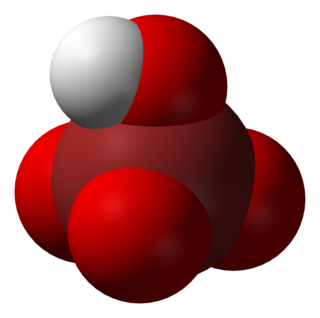
Perbromic acid is the inorganic compound with the formula HBrO4. Perbromic acid is characterized as a colorless liquid which has no characteristic scent. It is an oxoacid of bromine, with an oxidation state of 7+. Perbromic acid is a strong acid and strongly oxidizing, though dilute perbromic acid solutions are slow oxidizing agents. It is the most unstable of the halogen(VII) oxoacids. It decomposes rapidly on standing to bromic acid and oxygen, which releases toxic brown bromine vapors. It can be used in the synthesis of perbromate salts, by reacting with a base.
Selenium trioxide is the inorganic compound with the formula SeO3. It is white, hygroscopic solid. It is also an oxidizing agent and a Lewis acid. It is of academic interest as a precursor to Se(VI) compounds.

Selenium compounds are compounds containing the element selenium (Se). Among these compounds, selenium has various oxidation states, the most common ones being −2, +4, and +6. Selenium compounds exist in nature in the form of various minerals, such as clausthalite, guanajuatite, tiemannite, crookesite etc., and can also coexist with sulfide minerals such as pyrite and chalcopyrite. For many mammals, selenium compounds are essential. For example, selenomethionine and selenocysteine are selenium-containing amino acids present in the human body. Selenomethionine participates in the synthesis of selenoproteins. The reduction potential and pKa (5.47) of selenocysteine are lower than those of cysteine, making some proteins have antioxidant activity. Selenium compounds have important applications in semiconductors, glass and ceramic industries, medicine, metallurgy and other fields.
Bromine compounds are compounds containing the element bromine (Br). These compounds usually form the -1, +1, +3 and +5 oxidation states. Bromine is intermediate in reactivity between chlorine and iodine, and is one of the most reactive elements. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. This can be seen from the standard electrode potentials of the X2/X− couples (F, +2.866 V; Cl, +1.395 V; Br, +1.087 V; I, +0.615 V; At, approximately +0.3 V). Bromination often leads to higher oxidation states than iodination but lower or equal oxidation states to chlorination. Bromine tends to react with compounds including M–M, M–H, or M–C bonds to form M–Br bonds.
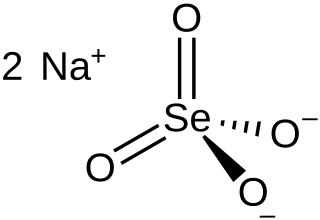
Sodium selenate is the inorganic compound with the formula Na
2SeO
4, not to be confused with sodium selenite. It exists as the anhydrous salt, the heptahydrate, and the decahydrate. These are white, water-soluble solids. The decahydrate is a common ingredient in multivitamins and livestock feed as a source of selenium. The anhydrous salt is used in the production of some glass. Although the selenates are much more toxic, many physical properties of sodium selenate and sodium sulfate are similar.

Sodium bismuthate is an inorganic compound, and a strong oxidiser with chemical formula NaBiO3. It is somewhat hygroscopic, but not soluble in cold water, which can be convenient since the reagent can be easily removed after the reaction. It is one of the few water insoluble sodium salts. Commercial samples may be a mixture of bismuth(V) oxide, sodium carbonate and sodium peroxide.

Potassium selenate, K
2SeO
4, is an odorless, white solid that forms as the potassium salt of selenic acid.
Iron(II) selenate (ferrous selenate) is an inorganic compound with the formula FeSeO4. It has anhydrous and several hydrate forms. The pentahydrate has the structure, [Fe(H2O)4]SeO4•H2O, isomorphous to the corresponding iron(II) sulfate. Heptahydrate is also known, in form of unstable green crystalline solid.
A selenate selenite is a chemical compound or salt that contains selenite and selenate anions (SeO32- and SeO42-). These are mixed anion compounds. Some have third anions.
Praseodymium(III) selenate is an inorganic compound, the salt of praseodymium and selenic acid with the chemical formula Pr2(SeO4)3. It forms green crystals when hydrated.

Erbium(III) selenate is an inorganic compound, with the chemical formula Er2(SeO4)3. It exists as an anhydrate or an octahydrate.



















