
A semimetal is a material with a very small overlap between the bottom of the conduction band and the top of the valence band. According to electronic band theory, solids can be classified as insulators, semiconductors, semimetals, or metals. In insulators and semiconductors the filled valence band is separated from an empty conduction band by a band gap. For insulators, the magnitude of the band gap is larger than that of a semiconductor. Because of the slight overlap between the conduction and valence bands, semimetals have no band gap and a negligible density of states at the Fermi level. A metal, by contrast, has an appreciable density of states at the Fermi level because the conduction band is partially filled.

A chalcogenide is a chemical compound consisting of at least one chalcogen anion and at least one more electropositive element. Although all group 16 elements of the periodic table are defined as chalcogens, the term chalcogenide is more commonly reserved for sulfides, selenides, tellurides, and polonides, rather than oxides. Many metal ores exist as chalcogenides. Photoconductive chalcogenide glasses are used in xerography. Some pigments and catalysts are also based on chalcogenides. The metal dichalcogenide MoS2 is a common solid lubricant.

Spin-density wave (SDW) and charge-density wave (CDW) are names for two similar low-energy ordered states of solids. Both these states occur at low temperature in anisotropic, low-dimensional materials or in metals that have high densities of states at the Fermi level . Other low-temperature ground states that occur in such materials are superconductivity, ferromagnetism and antiferromagnetism. The transition to the ordered states is driven by the condensation energy which is approximately where is the magnitude of the energy gap opened by the transition.
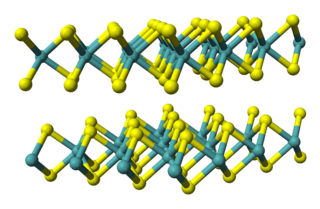
Tantalum(IV) sulfide is an inorganic compound with the formula TaS2. It is a layered compound with three-coordinate sulfide centres and trigonal prismatic or octahedral metal centres. It is structurally similar to molybdenum disulfide MoS2, and numerous other transition metal dichalcogenides. Tantalum disulfide has three polymorphs 1T-TaS2, 2H-TaS2, and 3R-TaS2, representing trigonal, hexagonal, and rhombohedral respectively.
Metal–insulator transitions are transitions of a material from a metal to an insulator. These transitions can be achieved by tuning various ambient parameters such as temperature, pressure or, in case of a semiconductor, doping.
A charge density wave (CDW) is an ordered quantum fluid of electrons in a linear chain compound or layered crystal. The electrons within a CDW form a standing wave pattern and sometimes collectively carry an electric current. The electrons in such a CDW, like those in a superconductor, can flow through a linear chain compound en masse, in a highly correlated fashion. Unlike a superconductor, however, the electric CDW current often flows in a jerky fashion, much like water dripping from a faucet due to its electrostatic properties. In a CDW, the combined effects of pinning and electrostatic interactions likely play critical roles in the CDW current's jerky behavior, as discussed in sections 4 & 5 below.

Niobium pentoxide is the inorganic compound with the formula Nb2O5. A colorless, insoluble, and fairly unreactive solid, it is the most widespread precursor for other compounds and materials containing niobium. It is predominantly used in alloying, with other specialized applications in capacitors, optical glasses, and the production of lithium niobate.
Alex K. Zettl is an American experimental physicist, educator, and inventor.
A Peierls transition or Peierls distortion is a distortion of the periodic lattice of a one-dimensional crystal. Atomic positions oscillate, so that the perfect order of the 1-D crystal is broken.

Niobium(V) bromide is the inorganic compound with the formula Nb2Br10. Its name comes from the compound's empirical formula, NbBr5. It is a diamagnetic, orange solid that hydrolyses readily. The compound adopts an edge-shared bioctahedral structure, which means that two NbBr5 units are joined by a pair of bromide bridges. There is no bond between the Nb centres. Niobium(V) chloride, niobium(V) iodide, tantalum(V) chloride, tantalum(V) bromide, and tantalum(V) iodide all share this structural motif.

Niobium(IV) fluoride is a chemical compound with the formula NbF4. It is a nonvolatile black solid.
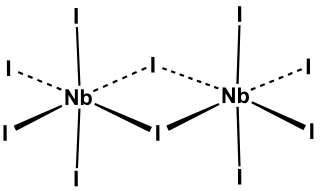
Niobium pentaiodide is the inorganic compound with the formula Nb2I10. Its name comes from the compound's empirical formula, NbI5. It is a diamagnetic, yellow solid that hydrolyses readily. The compound adopts an edge-shared bioctahedral structure, which means that two NbI5 units are joined by a pair of iodide bridges. There is no bond between the Nb centres. Niobium(V) chloride, niobium(V) bromide, tantalum(V) chloride, tantalum(V) bromide, and tantalum(V) iodide, all share this structural motif.

Niobium diselenide or niobium(IV) selenide is a layered transition metal dichalcogenide with formula NbSe2. Niobium diselenide is a lubricant, and a superconductor at temperatures below 7.2 K that exhibit a charge density wave (CDW). NbSe2 crystallizes in several related forms, and can be mechanically exfoliated into monatomic layers, similar to other transition metal dichalcogenide monolayers. Monolayer NbSe2 exhibits very different properties from the bulk material, such as of Ising superconductivity, quantum metallic state, and strong enhancement of the CDW.

Niobium disulfide is the chemical compound with the formula NbS2. It is a black layered solid that can be exfoliated into ultrathin grayish sheets similar to other transition metal dichalcogenides. These layers exhibit superconductivity, where the transition temperature increases from ca. 2 to 6 K with the layer thickness increasing from 6 to 12 nm, and then saturates with thickness.
Nai Phuan Ong is an American experimental physicist, specializing in "condensed matter physics focusing on topological insulators, Dirac/Weyl semimetals, superconductors and quantum spin liquids."
The selenide iodides are chemical compounds that contain both selenide ions (Se2−) and iodide ions (I−) and one or metal atoms. They are in the class of mixed anion compounds or chalcogenide halides.

Tantalum diselenide is a compound made with tantalum and selenium atoms, with chemical formula TaSe2, which belongs to the family of transition metal dichalcogenides. In contrast to molybdenum disulfide (MoS2) or rhenium disulfide (ReS2), tantalum diselenide does not occur spontaneously in nature, but it can be synthesized. Depending on the growth parameters, different types of crystal structures can be stabilized.
Jean Marcel Rouxel was a French synthetic chemist known for his work in solid state synthesis of low-dimensional materials. He pioneered the use of solid precursors in soft chemistry.
Ferecrystals (FCs) are a class of layered materials consisting of atomically thin layers of a transition metal dichalcogenides (TMDC) stacked alternately with metal monochalcogenide layers.
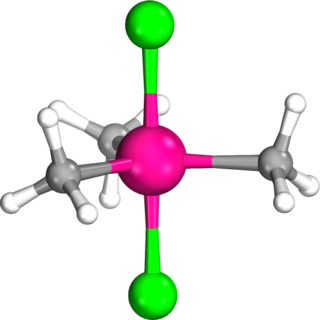
Dichlorotrimethyltantalum, or trimethyltantalum dichloride, is an organotantalum complex with the molecular formula TaCl2(CH3)3. It forms pale yellow, highly air- and water-sensitive crystals which easily sublime in vacuum at room temperature. It was the first reported σ-bonded alkyl complex of tantalum, synthesised by Gordon L. Juvinall at the California Institute of Technology in 1964. It serves as an important precursor for the preparation of a large number of Ta(V) complexes.













