| |
 | |
| Names | |
|---|---|
| IUPAC names Niobium(V) chloride Niobium pentachloride | |
| Identifiers | |
| |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.030.042 |
| EC Number |
|
PubChem CID | |
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) | |
| |
| Properties | |
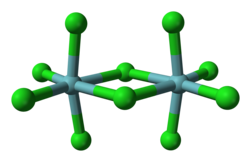
| NbCl5 | |
| Molar mass | 270.17 g/mol |
| Appearance | yellow monoclinic crystals deliquescent |
| Density | 2.75 g/cm3 |
| Melting point | 204.7 °C (400.5 °F; 477.8 K) |
| Boiling point | 248.2 °C (478.8 °F; 521.3 K) |
| decomposes | |
| Solubility | HCl, chloroform, CCl4 |
| Thermochemistry | |
Std molar entropy (S⦵298) | 214.05 J K−1 mol−1 |
Std enthalpy of formation (ΔfH⦵298) | −797.47 kJ/mol |
| Hazards | |
| GHS labelling: | |
  | |
| Danger | |
| H302, H312, H314, H332 | |
| P260, P261, P264, P270, P271, P280, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P321, P322, P330, P363, P405, P501 | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions | Niobium(V) fluoride Niobium(V) bromide Niobium(V) iodide |
Other cations | Vanadium(IV) chloride Tantalum(V) chloride |
Related niobium chlorides | Niobium(III) chloride Niobium(IV) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Niobium(V) chloride, also known as niobium pentachloride, is a yellow crystalline solid. It hydrolyzes in air, and samples are often contaminated with small amounts of NbOCl3. It is often used as a precursor to other compounds of niobium. NbCl5 may be purified by sublimation. [1]