
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name zirconium is derived from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian zargun. It is a lustrous, grey-white, strong transition metal that closely resembles hafnium and, to a lesser extent, titanium. Zirconium is mainly used as a refractory and opacifier, although small amounts are used as an alloying agent for its strong resistance to corrosion. Zirconium forms a variety of inorganic and organometallic compounds such as zirconium dioxide and zirconocene dichloride, respectively. Five isotopes occur naturally, four of which are stable. Zirconium compounds have no known biological role.
A Ziegler–Natta catalyst, named after Karl Ziegler and Giulio Natta, is a catalyst used in the synthesis of polymers of 1-alkenes (alpha-olefins). Two broad classes of Ziegler–Natta catalysts are employed, distinguished by their solubility:
Silane (Silicane) is an inorganic compound with chemical formula SiH4. It is a colourless, pyrophoric, toxic gas with a sharp, repulsive, pungent smell, somewhat similar to that of acetic acid. Silane is of practical interest as a precursor to elemental silicon. Silane with alkyl groups are effective water repellents for mineral surfaces such as concrete and masonry. Silanes with both organic and inorganic attachments are used as coupling agents. Silanes are commonly used to apply coatings to surfaces or as an adhesion promoter.

Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is a volatile liquid. Upon contact with humid air, it forms thick clouds of titanium dioxide and hydrochloric acid, a reaction that was formerly exploited for use in smoke machines. It is sometimes referred to as "tickle" or "tickle 4" due to the phonetic resemblance of its molecular formula to the word.

Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as the greenish tetrahydrate, which is the form that is most commonly encountered in commerce and the laboratory. There is also a dihydrate. The compound is highly soluble in water, giving pale green solutions.

Zirconium(IV) chloride, also known as zirconium tetrachloride, is an inorganic compound frequently used as a precursor to other compounds of zirconium. This white high-melting solid hydrolyzes rapidly in humid air.
Titanium(III) chloride is the inorganic compound with the formula TiCl3. At least four distinct species have this formula; additionally hydrated derivatives are known. TiCl3 is one of the most common halides of titanium and is an important catalyst for the manufacture of polyolefins.
Zirconium(IV) bromide is the inorganic compound with the formula ZrBr4. This colourless solid is the principal precursor to other Zr–Br compounds.
Technetium compounds are chemical compounds containing the chemical element technetium. Technetium can form multiple oxidation states, but often forms in the +4 and +7 oxidation states. Because technetium is radioactive, technetium compounds are extremely rare on Earth.

Organozirconium chemistry is the science of exploring the properties, structure, and reactivity of organozirconium compounds, which are organometallic compounds containing chemical bonds between carbon and zirconium. Organozirconium compounds have been widely studied, in part because they are useful catalysts in Ziegler-Natta polymerization.

Germanium dichloride is a chemical compound of germanium and chlorine with the formula GeCl2. It is a yellow solid. Germanium dichloride is an example of a compound featuring germanium in the +2 oxidation state.

Zirconium(III) chloride is an inorganic compound with formula ZrCl3. It is a blue-black solid that is highly sensitive to air.
In organometallic chemistry, bent metallocenes are a subset of metallocenes. In bent metallocenes, the ring systems coordinated to the metal are not parallel, but are tilted at an angle. A common example of a bent metallocene is Cp2TiCl2. Several reagents and much research is based on bent metallocenes.
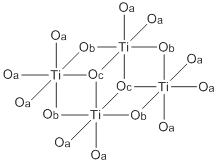
Titanium ethoxide is a chemical compound with the formula Ti4(OCH2CH3)16. It is a commercially available colorless liquid that is soluble in organic solvents but hydrolyzes readily. Alkoxides of titanium(IV) and zirconium(IV) are used in organic synthesis and materials science. They adopt more complex structures than suggested by their empirical formulas.

Metal halides are compounds between metals and halogens. Some, such as sodium chloride are ionic, while others are covalently bonded. A few metal halides are discrete molecules, such as uranium hexafluoride, but most adopt polymeric structures, such as palladium chloride.

Lead tetrachloride, also known as lead(IV) chloride, has the molecular formula PbCl4. It is a yellow, oily liquid which is stable below 0 °C, and decomposes at 50 °C. It has a tetrahedral configuration, with lead as the central atom. The Pb–Cl covalent bonds have been measured to be 247 pm and the bond energy is 243 kJ⋅mol−1.

Hafnocene dichloride is the organohafnium compound with the formula (C5H5)2HfCl2. It is a white solid that is sparingly soluble in some organic solvents. The lighter homologues zirconacene dichloride and titanocene dichloride have received much more attention. While hafnocene is only of academic interest, some more soluble derivatives are precatalysts for olefin polymerization. Moreso than the Zr analogue, this compound is highly resistant to reduction.

Protactinium(IV) chloride is an inorganic compound. It is an actinide halide, composed of protactinium and chlorine. It is radioactive, and has the chemical formula of PaCl4. It is a chartreuse-coloured (yellowish-green) crystal of the tetragonal crystal system.
Hafnium compounds are compounds containing the element hafnium (Hf). Due to the lanthanide contraction, the ionic radius of hafnium(IV) (0.78 ångström) is almost the same as that of zirconium(IV) (0.79 angstroms). Consequently, compounds of hafnium(IV) and zirconium(IV) have very similar chemical and physical properties. Hafnium and zirconium tend to occur together in nature and the similarity of their ionic radii makes their chemical separation rather difficult. Hafnium tends to form inorganic compounds in the oxidation state of +4. Halogens react with it to form hafnium tetrahalides. At higher temperatures, hafnium reacts with oxygen, nitrogen, carbon, boron, sulfur, and silicon. Some compounds of hafnium in lower oxidation states are known.













