Hafnium is a chemical element with the symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dmitri Mendeleev in 1869, though it was not identified until 1923, by Dirk Coster and George de Hevesy, making it the penultimate stable element to be discovered. Hafnium is named after Hafnia, the Latin name for Copenhagen, where it was discovered.

Zirconium is a chemical element with the symbol Zr and atomic number 40. The name zirconium is derived from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian zargun. It is a lustrous, grey-white, strong transition metal that closely resembles hafnium and, to a lesser extent, titanium. Zirconium is mainly used as a refractory and opacifier, although small amounts are used as an alloying agent for its strong resistance to corrosion. Zirconium forms a variety of inorganic and organometallic compounds such as zirconium dioxide and zirconocene dichloride, respectively. Five isotopes occur naturally, four of which are stable. Zirconium compounds have no known biological role.

Group 4 is the second group of transition metals in the periodic table. It contains the four elements titanium (Ti), zirconium (Zr), hafnium (Hf), and rutherfordium (Rf). The group is also called the titanium group or titanium family after its lightest member.

Hafnium(IV) chloride is the inorganic compound with the formula HfCl4. This colourless solid is the precursor to most hafnium organometallic compounds. It has a variety of highly specialized applications, mainly in materials science and as a catalyst.

Zirconium carbide (ZrC) is an extremely hard refractory ceramic material, commercially used in tool bits for cutting tools. It is usually processed by sintering.

Zirconium(IV) chloride, also known as zirconium tetrachloride, is an inorganic compound frequently used as a precursor to other compounds of zirconium. This white high-melting solid hydrolyzes rapidly in humid air.
Zirconium(IV) bromide is the inorganic compound with the formula ZrBr4. This colourless solid is the principal precursor to other Zr–Br compounds.

Zirconium(IV) fluoride describes members of a family inorganic compounds with the formula (ZrF4(H2O)x. All are colorless, diamagnetic solids. Anhydrous Zirconium(IV) fluoride' is a component of ZBLAN fluoride glass.

Borohydride refers to the anion [BH4]−, which is also called tetrahydroborate, and its salts. Borohydride or hydroborate is also the term used for compounds containing [BH4−nXn]−, where n is an integer from 0 to 3, for example cyanoborohydride or cyanotrihydroborate [BH3(CN)]− and triethylborohydride or triethylhydroborate [BH(CH2CH3)3]−. Borohydrides find wide use as reducing agents in organic synthesis. The most important borohydrides are lithium borohydride and sodium borohydride, but other salts are well known. Tetrahydroborates are also of academic and industrial interest in inorganic chemistry.

Organozirconium chemistry is the science of exploring the properties, structure, and reactivity of organozirconium compounds, which are organometallic compounds containing chemical bonds between carbon and zirconium. Organozirconium compounds have been widely studied, in part because they are useful catalysts in Ziegler-Natta polymerization.

Zirconium(III) chloride is an inorganic compound with formula ZrCl3. It is a blue-black solid that is highly sensitive to air.
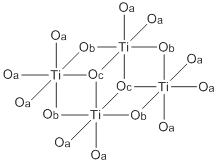
Titanium ethoxide is a chemical compound with the formula Ti4(OCH2CH3)16. It is a commercially available colorless liquid that is soluble in organic solvents but hydrolyzes readily. Alkoxides of titanium(IV) and zirconium(IV) are used in organic synthesis and materials science. They adopt more complex structures than suggested by their empirical formulas.

Germanium tetrafluoride (GeF4) is a chemical compound of germanium and fluorine. It is a colorless gas.

Hafnium acetylacetonate, also known as Hf(acac)4, is a coordination compound with formula Hf(C5H7O2)4. This white solid is the main hafnium complex of acetylacetonate. The complex has a square antiprismatic geometry with eight nearly equivalent Hf-O bonds. The molecular symmetry is D2, i.e., the complex is chiral. It is prepared from hafnium tetrachloride and acetylacetone, and base. Zr(acac)4 is very similar in structure and properties.

Zirconium nitrate is a volatile anhydrous transition metal nitrate salt of zirconium with formula Zr(NO3)4. It has alternate names of zirconium tetranitrate, or zirconium(IV) nitrate.

Hafnium disulfide is an inorganic compound of hafnium and sulfur. It is a layered dichalcogenide with the chemical formula is HfS2. A few atomic layers of this material can be exfoliated using the standard Scotch Tape technique (see graphene) and used for the fabrication of a field-effect transistor. High-yield synthesis of HfS2 has also been demonstrated using liquid phase exfoliation, resulting in the production of stable few-layer HfS2 flakes. Hafnium disulfide powder can be produced by reacting hydrogen sulfide and hafnium oxides at 500–1300 °C.

Zirconium(III) bromide is an inorganic compound with the formula ZrBr3.

Zirconium(III) iodide is an inorganic compound with the formula ZrI3.
Hafnium compounds are compounds containing the element hafnium (Hf). Due to the lanthanide contraction, the ionic radius of hafnium(IV) (0.78 ångström) is almost the same as that of zirconium(IV) (0.79 angstroms). Consequently, compounds of hafnium(IV) and zirconium(IV) have very similar chemical and physical properties. Hafnium and zirconium tend to occur together in nature and the similarity of their ionic radii makes their chemical separation rather difficult. Hafnium tends to form inorganic compounds in the oxidation state of +4. Halogens react with it to form hafnium tetrahalides. At higher temperatures, hafnium reacts with oxygen, nitrogen, carbon, boron, sulfur, and silicon. Some compounds of hafnium in lower oxidation states are known.

Hafnium(III) iodide is an inorganic compound of hafnium and iodine with the formula HfI3. It is a black solid.

















