The halogens are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would exclude tennessine as its chemistry is unknown and is theoretically expected to be more like that of gallium. In the modern IUPAC nomenclature, this group is known as group 17.

Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), produced as volatile derivatives of methane, ethane, and propane.

The haloalkanes are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes that contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula "RX" where R is an alkyl or substituted alkyl group and X is a halogen.

Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often have distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Several fluorocarbons and their derivatives are commercial polymers, refrigerants, drugs, and anesthetics.
A period 2 element is one of the chemical elements in the second row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases; a new row is started when chemical behavior begins to repeat, creating columns of elements with similar properties.

Halomethane compounds are derivatives of methane with one or more of the hydrogen atoms replaced with halogen atoms. Halomethanes are both naturally occurring, especially in marine environments, and human-made, most notably as refrigerants, solvents, propellants, and fumigants. Many, including the chlorofluorocarbons, have attracted wide attention because they become active when exposed to ultraviolet light found at high altitudes and destroy the Earth's protective ozone layer.

Hydrofluorocarbons (HFCs) are synthetic organic compounds that contain fluorine and hydrogen atoms, and are the most common type of organofluorine compounds. Most are gases at room temperature and pressure. They are frequently used in air conditioning and as refrigerants; R-134a (1,1,1,2-tetrafluoroethane) is one of the most commonly used HFC refrigerants. In order to aid the recovery of the stratospheric ozone layer, HFCs were adopted to replace the more potent chlorofluorocarbons (CFCs), which were phased out from use by the Montreal Protocol, and hydrochlorofluorocarbons (HCFCs) which are presently being phased out. HFCs replaced older chlorofluorocarbons such as R-12 and hydrochlorofluorocarbons such as R-21. HFCs are also used in insulating foams, aerosol propellants, as solvents and for fire protection.
1,1-Difluoroethane, or DFE, is an organofluorine compound with the chemical formula C2H4F2. This colorless gas is used as a refrigerant, where it is often listed as R-152a (refrigerant-152a) or HFC-152a (hydrofluorocarbon-152a). It is also used as a propellant for aerosol sprays and in gas duster products. As an alternative to chlorofluorocarbons, it has an ozone depletion potential of zero, a lower global warming potential (124) and a shorter atmospheric lifetime (1.4 years).

Oxygen fluorides are compounds of elements oxygen and fluorine with the general formula OnF2, where n = 1 to 6. Many different oxygen fluorides are known:

Dioxygen difluoride is a compound of fluorine and oxygen with the molecular formula O2F2. It can exist as an orange-red colored solid which melts into a red liquid at −163 °C (110 K). It is an extremely strong oxidant and decomposes into oxygen and fluorine even at −160 °C (113 K) at a rate of 4% per day — its lifetime at room temperature is thus extremely short. Dioxygen difluoride reacts vigorously with nearly every chemical it encounters (including ordinary ice) leading to its onomatopoeic nickname FOOF (a play on its chemical structure and its explosive tendencies).

Tetrafluoromethane, also known as carbon tetrafluoride or R-14, is the simplest perfluorocarbon (CF4). As its IUPAC name indicates, tetrafluoromethane is the perfluorinated counterpart to the hydrocarbon methane. It can also be classified as a haloalkane or halomethane. Tetrafluoromethane is a useful refrigerant but also a potent greenhouse gas. It has a very high bond strength due to the nature of the carbon–fluorine bond.
Fluoroform, or trifluoromethane, is the chemical compound with the formula CHF3. It is a hydrofluorocarbon as well as being a part of the haloforms, a class of compounds with the formula CHX3 with C3v symmetry. Fluoroform is used in diverse applications in organic synthesis. It is not an ozone depleter but is a greenhouse gas.
Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents.

The carbon–fluorine bond is a polar covalent bond between carbon and fluorine that is a component of all organofluorine compounds. It is one of the strongest single bonds in chemistry, and relatively short, due to its partial ionic character. The bond also strengthens and shortens as more fluorines are added to the same carbon on a chemical compound. As such, fluoroalkanes like tetrafluoromethane are some of the most unreactive organic compounds.

Fluorine is a chemical element; it has symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as pale yellow diatomic gas. Fluorine is extremely reactive as it reacts with all other elements except for the light inert gases. It is highly toxic.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.

Fluorine may interact with biological systems in the form of fluorine-containing compounds. Though elemental fluorine (F2) is very rare in everyday life, fluorine-containing compounds such as fluorite occur naturally as minerals. Naturally occurring organofluorine compounds are extremely rare. Man-made fluoride compounds are common and are used in medicines, pesticides, and materials. Twenty percent of all commercialized pharmaceuticals contain fluorine, including Lipitor and Prozac. In many contexts, fluorine-containing compounds are harmless or even beneficial to living organisms; in others, they are toxic.
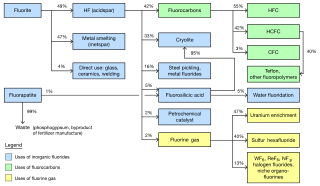
The global market for chemicals from fluorine was about US$16 billion per year as of 2006. The industry was predicted to reach 2.6 million metric tons per year by 2015. The largest market is the United States. Western Europe is the second largest. Asia Pacific is the fastest growing region of production. China in particular has experienced significant growth as a fluorochemical market and is becoming a producer of them as well. Fluorite mining was estimated in 2003 to be a $550 million industry, extracting 4.5 million tons per year.

Fluorine is a relatively new element in human applications. In ancient times, only minor uses of fluorine-containing minerals existed. The industrial use of fluorite, fluorine's source mineral, was first described by early scientist Georgius Agricola in the 16th century, in the context of smelting. The name "fluorite" derives from Agricola's invented Latin terminology. In the late 18th century, hydrofluoric acid was discovered. By the early 19th century, it was recognized that fluorine was a bound element within compounds, similar to chlorine. Fluorite was determined to be calcium fluoride.
1,2-Difluoroethane is a saturated hydrofluorocarbon containing an atom of fluorine attached to each of two carbons atoms. The formula can be written CH2FCH2F. It is an isomer of 1,1-difluoroethane. It has a HFC name of HFC-152 with no letter suffix. When cooled to cryogenic temperatures it can have different conformers, gauche and trans. In the liquid form these are about equally abundant and easily interconvert. As a gas it is mostly the gauche form.