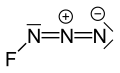| |||
| Names | |||
|---|---|---|---|
| Other names triazadienyl fluoride | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChemSpider | |||
PubChem CID | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| FN3 | |||
| Molar mass | 61.019 g/mol | ||
| Appearance | Yellow-green gas | ||
| Density | 1.3 g/cm3 [1] | ||
| Melting point | −139 °C (−218 °F; 134 K) [2] | ||
| Boiling point | −30 °C (−22 °F; 243 K) [2] | ||
| Explosive data | |||
| Shock sensitivity | Extreme | ||
| Friction sensitivity | Extreme | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards | Extremely sensitive explosive | ||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Other cations | Hydrazoic acid Chlorine azide Bromine azide Iodine azide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Fluorine azide or triazadienyl fluoride is a yellow green gas composed of nitrogen and fluorine with formula FN3. [3] Its properties resemble those of ClN3, BrN3, and IN3. [4] The bond between the fluorine atom and the nitrogen is very weak, leading to this substance being very unstable and prone to explosion. [5] Calculations show the F–N–N angle to be around 102° with a straight line of 3 nitrogen atoms. [6]
Contents
It was first made by John F. Haller in 1942. [7]