 | |
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID | |
| |
| |
| Properties | |
| F5Os | |
| Molar mass | 285.22 g·mol−1 |
| Appearance | blue-green solid |
| Melting point | 70 °C (158 °F; 343 K) |
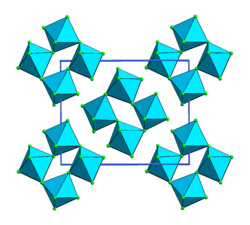
| Structure [1] | |
| Monoclinic | |
| P21/c (No. 14) | |
a = 5.53 Å, b = 9.91 Å, c = 12.59 Å α = 90°, β = 99.5°, γ = 90° | |
Lattice volume (V) | 680 Å3 |
Formula units (Z) | 8 units per cell |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
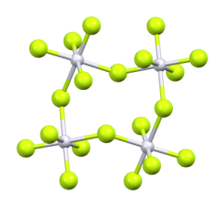
Osmium pentafluoride is an inorganic compound with the formula OsF5. It is a blue-green solid. Like the pentafluorides of Ru, Rh, and Ir, OsF5 exists as a tetramer in the solid state.