In chemistry, a halide is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, astatide, or theoretically tennesside compound. The alkali metals combine directly with halogens under appropriate conditions forming halides of the general formula, MX. Many salts are halides; the hal- syllable in halide and halite reflects this correlation. All Group 1 metals form halides that are white solids at room temperature.

Beryllium fluoride is the inorganic compound with the formula BeF2. This white solid is the principal precursor for the manufacture of beryllium metal. Its structure resembles that of quartz, but BeF2 is highly soluble in water.

Plutonium(IV) fluoride is a chemical compound with the formula PuF4. This salt is generally a brown solid but can appear a variety of colors depending on the grain size, purity, moisture content, lighting, and presence of contaminants. Its primary use in the United States has been as an intermediary product in the production of plutonium metal for nuclear weapons usage.

Tellurium tetrafluoride, TeF4, is a stable, white, hygroscopic crystalline solid and is one of two fluorides of tellurium. The other binary fluoride is tellurium hexafluoride. The widely reported Te2F10 has been shown to be F5TeOTeF5 There are other tellurium compounds that contain fluorine, but only the two mentioned contain solely tellurium and fluorine. Tellurium difluoride, TeF2, and ditellurium difluoride, Te2F2 are not known.

Zirconium(IV) fluoride describes members of a family inorganic compounds with the formula ZrF4(H2O)x. All are colorless, diamagnetic solids. Anhydrous Zirconium(IV) fluoride is a component of ZBLAN fluoride glass.

Selenium tetrafluoride (SeF4) is an inorganic compound. It is a colourless liquid that reacts readily with water. It can be used as a fluorinating reagent in organic syntheses (fluorination of alcohols, carboxylic acids or carbonyl compounds) and has advantages over sulfur tetrafluoride in that milder conditions can be employed and it is a liquid rather than a gas.
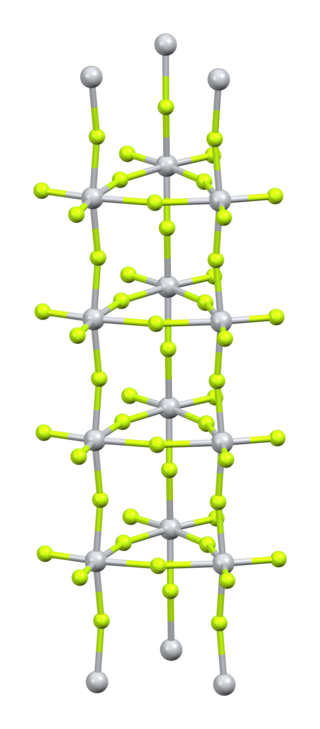
Titanium(IV) fluoride is the inorganic compound with the formula TiF4. It is a white hygroscopic solid. In contrast to the other tetrahalides of titanium, it adopts a polymeric structure. In common with the other tetrahalides, TiF4 is a strong Lewis acid.

Manganese tetrafluoride, MnF4, is the highest fluoride of manganese. It is a powerful oxidizing agent and is used as a means of purifying elemental fluorine.
Arsenic trifluoride is a chemical compound of arsenic and fluorine with the chemical formula AsF3. It is a colorless liquid which reacts readily with water. Like other inorganic arsenic compounds, it is highly toxic.

Bismuth(III) fluoride or bismuth trifluoride is a chemical compound of bismuth and fluorine. The chemical formula is BiF3. It is a grey-white powder melting at 649 °C.

Iridium(IV) fluoride is a chemical compound of iridium and fluorine, with the chemical formula IrF4 and is a dark brown solid. Early reports of IrF4 prior to 1965 are questionable and appear to describe the compound IrF5. The solid can be prepared by reduction of IrF5 with iridium black or reduction with H2 in aqueous HF. The crystal structure of the solid is notable as it was the first example of a three-dimensional lattice structure found for a metal tetrafluoride and subsequently RhF4, PdF4 and PtF4 have been found to have the same structure. The structure has 6 coordinate, octahedral, iridium where two edges of the octahedra are shared and the two unshared fluorine atoms are cis to one another.

Tin(IV) fluoride is a chemical compound of tin and fluorine with the chemical formula SnF4 and is a white solid with a melting point above 700 °C.

Niobium(IV) fluoride is a chemical compound with the formula NbF4. It is a nonvolatile black solid.

Neptunium(VI) fluoride (NpF6) is the highest fluoride of neptunium, it is also one of seventeen known binary hexafluorides. It is a volatile orange crystalline solid. It is relatively hard to handle, being very corrosive, volatile and radioactive. Neptunium hexafluoride is stable in dry air but reacts vigorously with water.

Chromium(IV) fluoride is an inorganic compound with the chemical formula CrF4. It has a dark greenish-black color when solid. It rapidly hydrolyzes in presence of moisture in air or directly in water.
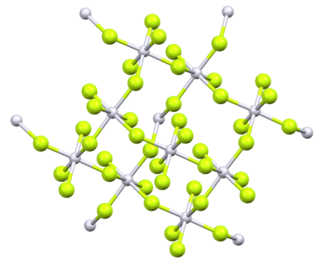
Platinum tetrafluoride is the inorganic compound with the chemical formula PtF
4. In the solid state, the compound features platinum(IV) in octahedral coordination geometry.
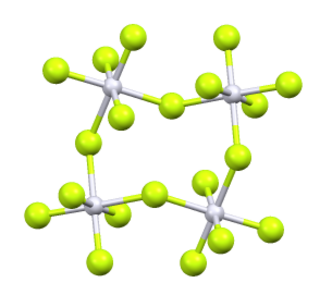
Platinum pentafluoride is the inorganic compound with the empirical formula PtF5. This red volatile solid has rarely been studied but is of interest as one of the few binary fluorides of platinum, i.e., a compound containing only Pt and F. It is hydrolyzed in water.
Gadolinium(III) fluoride is an inorganic compound with a chemical formula GdF3.

Terbium(IV) fluoride is an inorganic compound with a chemical formula TbF4. It is a white solid that is a strong oxidizer. It is also a strong fluorinating agent, emitting relatively pure atomic fluorine when heated, rather than the mixture of fluoride vapors emitted from cobalt(III) fluoride or cerium(IV) fluoride.
Americium compounds are compounds containing the element americium (Am). These compounds can form in the +2, +3, and +4, although the +3 oxidation state is the most common. The +5, +6 and +7 oxidation states have also been reported.














