
Protactinium is a chemical element; it has symbol Pa and atomic number 91. It is a dense, radioactive, silvery-gray actinide metal which readily reacts with oxygen, water vapor, and inorganic acids. It forms various chemical compounds, in which protactinium is usually present in the oxidation state +5, but it can also assume +4 and even +3 or +2 states. Concentrations of protactinium in the Earth's crust are typically a few parts per trillion, but may reach up to a few parts per million in some uraninite ore deposits. Because of its scarcity, high radioactivity, and high toxicity, there are currently no uses for protactinium outside scientific research, and for this purpose, protactinium is mostly extracted from spent nuclear fuel.

Titanium tetraiodide is an inorganic compound with the formula TiI4. It is a black volatile solid, first reported by Rudolph Weber in 1863. It is an intermediate in the van Arkel–de Boer process for the purification of titanium.

Silicon tetraiodide is the chemical compound with the formula SiI4. It is a tetrahedral molecule with Si-I bond lengths of 2.432(5) Å.

Tellurium tetraiodide (TeI4) is an inorganic chemical compound. It has a tetrameric structure which is different from the tetrameric solid forms of TeCl4 and TeBr4. In TeI4 the Te atoms are octahedrally coordinated and edges of the octahedra are shared.
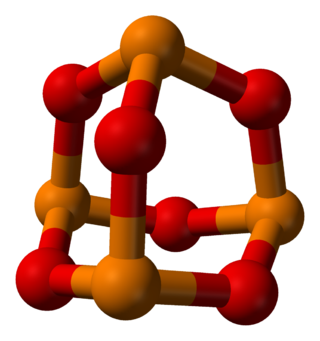
Phosphorus trioxide is the chemical compound with the molecular formula P4O6. Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. This colorless solid is structurally related to adamantane. It is formally the anhydride of phosphorous acid, H3PO3, but cannot be obtained by the dehydration of the acid. A white solid that melts at room temperature, it is waxy, crystalline and highly toxic, with garlic odor.

Organoactinide chemistry is the science exploring the properties, structure, and reactivity of organoactinide compounds, which are organometallic compounds containing a carbon to actinide chemical bond.

Bismuth(III) iodide is the inorganic compound with the formula BiI3. This gray-black salt is the product of the reaction of bismuth and iodine, which once was of interest in qualitative inorganic analysis.
Polysilicon halides are silicon-backbone polymeric solids. At room temperature, the polysilicon fluorides are colorless to yellow solids while the chlorides, bromides, and iodides are, respectively, yellow, amber, and red-orange. Polysilicon dihalides (perhalo-polysilenes) have the general formula (SiX2)n while the polysilicon monohalides (perhalo-polysilynes) have the formula (SiX)n, where X is F, Cl, Br, or I and n is the number of monomer units in the polymer.
Polyhalogen ions are a group of polyatomic cations and anions containing halogens only. The ions can be classified into two classes, isopolyhalogen ions which contain one type of halogen only, and heteropolyhalogen ions with more than one type of halogen.

Many compounds of thorium are known: this is because thorium and uranium are the most stable and accessible actinides and are the only actinides that can be studied safely and legally in bulk in a normal laboratory. As such, they have the best-known chemistry of the actinides, along with that of plutonium, as the self-heating and radiation from them is not enough to cause radiolysis of chemical bonds as it is for the other actinides. While the later actinides from americium onwards are predominantly trivalent and behave more similarly to the corresponding lanthanides, as one would expect from periodic trends, the early actinides up to plutonium have relativistically destabilised and hence delocalised 5f and 6d electrons that participate in chemistry in a similar way to the early transition metals of group 3 through 8: thus, all their valence electrons can participate in chemical reactions, although this is not common for neptunium and plutonium.
Polonium tetraiodide is a binary inorganic compound of polonium and iodine with the chemical formula PoI
4. The compound forms volatile black crystals.
Protactinium(V) iodide is an inorganic compound, with the chemical formula of PaI5.
Protactinium(V) bromide is an inorganic compound. It is a halide of protactinium, consisting of protactinium and bromine. It is radioactive and has a chemical formula of PaBr5, which is a red crystal of the monoclinic crystal system.

Protactinium(IV) chloride is an inorganic compound. It is an actinide halide, a salt composed of protactinium and chlorine. It is radioactive, and has the chemical formula of PaCl4. It is a chartreuse-coloured (yellowish-green) crystal of the tetragonal crystal system.

Protactinium(IV) bromide is an inorganic compound. It is an actinide halide, composed of protactinium and bromine. It is radioactive, and has the chemical formula of PaBr4. It may be due to the brown color of bromine that causes the appearance of protactinium(IV) bromide to be brown crystals. Its crystal structure is tetragonal. Protactinium(IV) bromide is sublimed in a vacuum at 400 °C. The protactinium(IV) halide closest in structure to protactinium(IV) bromide is protactinium(IV) chloride.
Europium(III) iodide is an inorganic compound containing europium and iodine with the chemical formula EuI3.
Americium compounds are compounds containing the element americium (Am). These compounds can form in the +2, +3, and +4, although the +3 oxidation state is the most common. The +5, +6 and +7 oxidation states have also been reported.
Rhenium tetraiodide is a binary chemical compound of rhenium and iodide with the chemical formula ReI
4.

Protactinium tetrafluoride is a binary inorganic compound of protactinium metal and fluorine with the chemical formula PaF4.
Thorium triiodide is a binary inorganic compound of thorium metal and iodine with the chemical formula ThI3.








