
Titanium tetraiodide is an inorganic compound with the formula TiI4. It is a black volatile solid, first reported by Rudolph Weber in 1863. It is an intermediate in the van Arkel–de Boer process for the purification of titanium.

Thallium triiodide is a chemical compound of thallium and iodine with formula TlI3. Unlike the other thallium trihalides, which contain thallium(III), TlI3 is a thallium(I) salt and contains the triiodide ion, I−
3.
Cerium(III) iodide (CeI3) is the compound formed by cerium(III) cations and iodide anions.
Iron(III) iodide is an inorganic compound with the chemical formula FeI3. It is a thermodynamically unstable compound that is difficult to prepare. Nevertheless, iron(III) iodide has been synthesised in small quantities in the absence of air and water.

Neodymium(II) iodide or neodymium diiodide is an inorganic salt of iodine and neodymium the formula NdI2. Neodymium uses the +2 oxidation state in the compound.

Praseodymium(III) iodide is an inorganic salt, consisting of the rare-earth metal praseodymium and iodine, with the chemical formula PrI3. It forms green crystals. It is soluble in water.

Praseodymium diiodide is a chemical compound with the empirical formula of PrI2, consisting of praseodymium and iodine. It is an electride, with the ionic formula of Pr3+(I−)2e−, and therefore not a true praseodymium(II) compound.

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Many europium compounds fluoresce under ultraviolet light due to the excitation of electrons to higher energy levels. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.

Lanthanum(III) iodide is an inorganic compound containing lanthanum and iodine with the chemical formula LaI
3.
Europium(III) iodide is an inorganic compound containing europium and iodine with the chemical formula EuI3.

Lutetium(III) iodide or lutetium iodide is an inorganic compound consisting of iodine and lutetium, with the chemical formula of LuI3.

Gadolinium(III) iodide is an iodide of gadolinium, with the chemical formula of GdI3. It is a yellow, highly hygroscopic solid with a bismuth(III) iodide-type crystal structure. In air, it quickly absorbs moisture and forms hydrates. The corresponding oxide iodide is also readily formed at elevated temperature.

Gadolinium diiodide is an inorganic compound, with the chemical formula of GdI2. It is an electride, with the ionic formula of Gd3+(I−)2e−, and therefore not a true gadolinium(II) compound. It is ferromagnetic at 276 K with a saturation magnetization of 7.3 B; it exhibits a large negative magnetoresistance (~70%) at 7 T near room temperature. It can be obtained by reacting gadolinium and gadolinium(III) iodide at a high temperature:

Thulium(III) iodide is an iodide of thulium, with the chemical formula of TmI3. Thulium(III) iodide is used as a component of metal halide lamps.

Lanthanum diiodide is an iodide of lanthanum, with the chemical formula of LaI2. It is an electride, actually having a chemical formula of La3+[(I−)2e−].

Holmium(III) iodide is an iodide of holmium, with the chemical formula of HoI3. It is used as a component of metal halide lamps.
Lutetium compounds are compounds formed by the lanthanide metal lutetium (Lu). In these compounds, lutetium generally exhibits the +3 oxidation state, such as LuCl3, Lu2O3 and Lu2(SO4)3. Aqueous solutions of most lutetium salts are colorless and form white crystalline solids upon drying, with the common exception of the iodide. The soluble salts, such as nitrate, sulfate and acetate form hydrates upon crystallization. The oxide, hydroxide, fluoride, carbonate, phosphate and oxalate are insoluble in water.
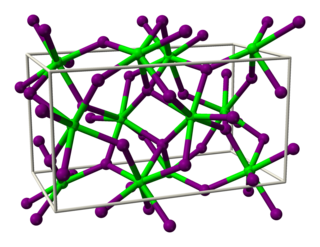
Thulium dibromide is an inorganic compound, with the chemical formula of TmBr2. It is a dark green solid that is easy to dissolve, with the SrI2 structure and it needs to be stored in an inert atmosphere.

Ytterbium(III) iodide is one of ytterbium's iodides, with the chemical formula of YbI3.

Plutonium(III) iodide is the iodide of plutonium with the chemical formula PuI3.









