In chemistry, an interhalogen compound is a molecule which contains two or more different halogen atoms and no atoms of elements from any other group.

Phosphorus triiodide (PI3) is an inorganic compound with the formula PI3. A red solid, it is too unstable to be stored; it is, nevertheless, commercially available. It is widely used in organic chemistry for converting alcohols to alkyl iodides. It is also a powerful reducing agent. Note that phosphorus also forms a lower iodide, P2I4, but the existence of PI5 is doubtful at room temperature.

Titanium tetraiodide is an inorganic compound with the formula TiI4. It is a black volatile solid, first reported by Rudolph Weber in 1863. It is an intermediate in the van Arkel–de Boer process for the purification of titanium.

Carbon tetraiodide is a tetrahalomethane with the molecular formula CI4. Being bright red, it is a relatively rare example of a highly colored methane derivative. It is only 2.3% by weight carbon, although other methane derivatives are known with still less carbon.
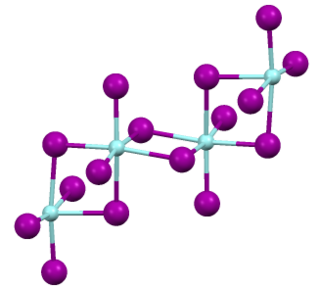
Zirconium(IV) iodide is the chemical compound with the formula ZrI4. It is the most readily available iodide of zirconium. It is an orange-coloured solid that degrades in the presence of water. The compound was once prominent as an intermediate in the purification of zirconium metal.

Silicon tetraiodide is the chemical compound with the formula SiI4. It is a tetrahedral molecule with Si-I bond lengths of 2.432(5) Å.

Tungsten(IV) oxide is the chemical compound with the formula WO2. The bronze-colored solid crystallizes in a monoclinic cell. The rutile-like structure features distorted octahedral WO6 centers with alternate short W–W bonds (248 pm). Each tungsten center has the d2 configuration, which gives the material a high electrical conductivity.

Tellurium tetraiodide (TeI4) is an inorganic chemical compound. It has a tetrameric structure which is different from the tetrameric solid forms of TeCl4 and TeBr4. In TeI4 the Te atoms are octahedrally coordinated and edges of the octahedra are shared.

Technetium hexafluoride or technetium(VI) fluoride (TcF6) is a yellow inorganic compound with a low melting point. It was first identified in 1961. In this compound, technetium has an oxidation state of +6, the highest oxidation state found in the technetium halides. In this respect, technetium differs from rhenium, which forms a heptafluoride, ReF7. Technetium hexafluoride occurs as an impurity in uranium hexafluoride, as technetium is a fission product of uranium (spontaneous fission in natural uranium, possible contamination from induced fission inside the reactor in reprocessed uranium). The fact that the boiling point of the hexafluorides of uranium and technetium are very close to each other presents a problem in using fluoride volatility in nuclear reprocessing.
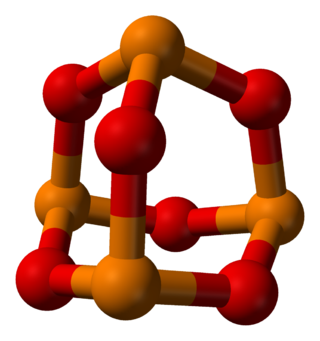
Phosphorus trioxide is the chemical compound with the molecular formula P4O6. Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. This colorless solid is structurally related to adamantane. It is formally the anhydride of phosphorous acid, H3PO3, but cannot be obtained by the dehydration of the acid. A white solid that melts at room temperature, it is waxy, crystalline and highly toxic, with garlic odor.
Tin(IV) iodide, also known as stannic iodide, is the chemical compound with the formula SnI4. This tetrahedral molecule crystallizes as a bright orange solid that dissolves readily in nonpolar solvents such as benzene.

Diarsenic tetraiodide is an inorganic compound of arsenic and iodine. It is a dark red metastable solid. The compound is a closely related to the better characterized diphosphorus tetraiodide. Identified in the late 19th century with the (accurate) empirical formula AsI2, the compound was assigned the formula (As2I4) several years later.

Iodosilane is a chemical compound of silicon, hydrogen, and iodine. It is a colorless monoclinic crystal of space group P21/c at −157 °C.

Germanium(IV) iodide is an inorganic compound with the chemical formula GeI4.

Praseodymium(IV) fluoride (also praseodymium tetrafluoride) is a binary inorganic compound, a highly oxidised metal salt of praseodymium and fluoride with the chemical formula PrF4.

Germanium tetrabromide is the inorganic compound with the formula GeBr4. It is a colorless solid that melts near room temperature. It can be formed by treating solid germanium with bromine, or by treating a silicon-copper mixture with bromine:
Polonium tetraiodide is a binary inorganic compound of polonium and iodine with the chemical formula PoI
4. The compound forms volatile black crystals.

Berkelium(III) chloride also known as berkelium trichloride, is a chemical compound with the formula BkCl3. It is a water-soluble green salt with a melting point of 603 °C. This compound forms the hexahydrate, BkCl3·6H2O.
Protactinium(V) iodide is an inorganic compound, with the chemical formula of PaI5.

Protactinium(IV) chloride is an inorganic compound. It is an actinide halide, composed of protactinium and chlorine. It is radioactive, and has the chemical formula of PaCl4. It is a chartreuse-coloured (yellowish-green) crystal of the tetragonal crystal system.


















