Iron(III) chloride describes the inorganic compounds with the formula FeCl3(H2O)x. Also called ferric chloride, these compounds are some of the most important and commonplace compounds of iron. They are available both in anhydrous and in hydrated forms, which are both hygroscopic. They feature iron in its +3 oxidation state. The anhydrous derivative is a Lewis acid, while all forms are mild oxidizing agents. It is used as a water cleaner and as an etchant for metals.

Copper(II) nitrate describes any member of the family of inorganic compounds with the formula Cu(NO3)2(H2O)x. The hydrates are hygroscopic blue solids. Anhydrous copper nitrate forms blue-green crystals and sublimes in a vacuum at 150-200 °C. Common hydrates are the hemipentahydrate and trihydrate.

Zinc chloride is an inorganic chemical compound with the formula ZnCl2·nH2O, with n ranging from 0 to 4.5, forming hydrates. Zinc chloride, anhydrous and its hydrates, are colorless or white crystalline solids, and are highly soluble in water. Five hydrates of zinc chloride are known, as well as four forms of anhydrous zinc chloride.
In chemistry, water(s) of crystallization or water(s) of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. In some contexts, water of crystallization is the total mass of water in a substance at a given temperature and is mostly present in a definite (stoichiometric) ratio. Classically, "water of crystallization" refers to water that is found in the crystalline framework of a metal complex or a salt, which is not directly bonded to the metal cation.

Manganese(II) chloride is the dichloride salt of manganese, MnCl2. This inorganic chemical exists in the anhydrous form, as well as the dihydrate (MnCl2·2H2O) and tetrahydrate (MnCl2·4H2O), with the tetrahydrate being the most common form. Like many Mn(II) species, these salts are pink, with the paleness of the color being characteristic of transition metal complexes with high spin d5 configurations.

Cobalt(II) chloride is an inorganic compound, a salt of cobalt and chlorine, with the formula CoCl
2. The compound forms several hydrates CoCl
2·nH
2O, for n = 1, 2, 6, and 9. Claims of the formation of tri- and tetrahydrates have not been confirmed. The anhydrous form is a blue crystalline solid; the dihydrate is purple and the hexahydrate is pink. Commercial samples are usually the hexahydrate, which is one of the most commonly used cobalt salts in the lab.

Chromium(III) chloride (also called chromic chloride) is an inorganic chemical compound with the chemical formula CrCl3. It forms several hydrates with the formula CrCl3·nH2O, among which are hydrates where n can be 5 (chromium(III) chloride pentahydrate CrCl3·5H2O) or 6 (chromium(III) chloride hexahydrate CrCl3·6H2O). The anhydrous compound with the formula CrCl3 are violet crystals, while the most common form of the chromium(III) chloride are the dark green crystals of hexahydrate, CrCl3·6H2O. Chromium chlorides find use as catalysts and as precursors to dyes for wool.

Nickel(II) chloride (or just nickel chloride) is the chemical compound NiCl2. The anhydrous salt is yellow, but the more familiar hydrate NiCl2·6H2O is green. Nickel(II) chloride, in various forms, is the most important source of nickel for chemical synthesis. The nickel chlorides are deliquescent, absorbing moisture from the air to form a solution. Nickel salts have been shown to be carcinogenic to the lungs and nasal passages in cases of long-term inhalation exposure.
Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as the greenish tetrahydrate, which is the form that is most commonly encountered in commerce and the laboratory. There is also a dihydrate. The compound is highly soluble in water, giving pale green solutions.

Cadmium chloride is a white crystalline compound of cadmium and chloride, with the formula CdCl2. This salt is a hygroscopic solid that is highly soluble in water and slightly soluble in alcohol. The crystal structure of cadmium chloride (described below), is a reference for describing other crystal structures. Also known are CdCl2•H2O and the hemipentahydrate CdCl2•2.5H2O.

Rhodium(III) chloride refers to inorganic compounds with the formula RhCl3(H2O)n, where n varies from 0 to 3. These are diamagnetic red-brown solids. The soluble trihydrated (n = 3) salt is the usual compound of commerce. It is widely used to prepare compounds used in homogeneous catalysis.

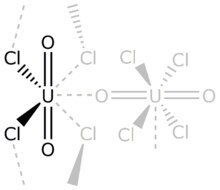
Uranium tetrachloride is an inorganic compound, a salt of uranium and chlorine, with the formula UCl4. It is a hygroscopic olive-green solid. It was used in the electromagnetic isotope separation (EMIS) process of uranium enrichment. It is one of the main starting materials for organouranium chemistry.

Uranyl fluoride is the inorganic compound with the formula UO2F2. It is most notable as a contaminant in the production of uranium tetrafluoride.
Titanium(III) chloride is the inorganic compound with the formula TiCl3. At least four distinct species have this formula; additionally hydrated derivatives are known. TiCl3 is one of the most common halides of titanium and is an important catalyst for the manufacture of polyolefins.

Vanadyl(IV) sulfate describes a collection of inorganic compounds of vanadium with the formula, VOSO4(H2O)x where 0 ≤ x ≤ 6. The pentahydrate is common. This hygroscopic blue solid is one of the most common sources of vanadium in the laboratory, reflecting its high stability. It features the vanadyl ion, VO2+, which has been called the "most stable diatomic ion".

Iron(III) nitrate, or ferric nitrate, is the name used for a series of inorganic compounds with the formula Fe(NO3)3.(H2O)n. Most common is the nonahydrate Fe(NO3)3.(H2O)9. The hydrates are all pale colored, water-soluble paramagnetic salts.

Chloroauric acid is an inorganic compound with the chemical formula H[AuCl4]. It forms hydrates H[AuCl4]·nH2O. Both the trihydrate and tetrahydrate are known. Both are orange-yellow solids consisting of the planar [AuCl4]− anion. Often chloroauric acid is handled as a solution, such as those obtained by dissolution of gold in aqua regia. These solutions can be converted to other gold complexes or reduced to metallic gold or gold nanoparticles.

Metal halides are compounds between metals and halogens. Some, such as sodium chloride are ionic, while others are covalently bonded. A few metal halides are discrete molecules, such as uranium hexafluoride, but most adopt polymeric structures, such as palladium chloride.

Nickel(II) perchlorate is a collection of inorganic compounds with the chemical formula of Ni(ClO4)2(H2O)x. Its colors of these solids vary with the degree of hydration. For example, the hydrate forms cyan crystals, the pentahydrate forms green crystals, but the hexahydrate (Ni(ClO4)2·6H2O) forms blue crystals. Nickel(II) perchlorate hexahydrate is highly soluble in water and soluble in some polar organic solvents.

In chemistry, a transition metal ether complex is a coordination complex consisting of a transition metal bonded to one or more ether ligand. The inventory of complexes is extensive. Common ether ligands are diethyl ether and tetrahydrofuran. Common chelating ether ligands include the glymes, dimethoxyethane (dme) and diglyme, and the crown ethers. Being lipophilic, metal-ether complexes often exhibit solubility in organic solvents, a property of interest in synthetic chemistry. In contrast, the di-ether 1,4-dioxane is generally a bridging ligand.