
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of group trends in properties in the periodic table, with elements exhibiting well-characterised homologous behaviour. This family of elements is also known as the lithium family after its leading element.

Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li[AlH4] or LiAlH4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides. The solid is dangerously reactive toward water, releasing gaseous hydrogen (H2). Some related derivatives have been discussed for hydrogen storage.

In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric.

Zinc chloride is an inorganic chemical compound with the formula ZnCl2·nH2O, with n ranging from 0 to 4.5, forming hydrates. Zinc chloride, anhydrous and its hydrates, are colorless or white crystalline solids, and are highly soluble in water. Five hydrates of zinc chloride are known, as well as four forms of anhydrous zinc chloride.
The Simmons–Smith reaction is an organic cheletropic reaction involving an organozinc carbenoid that reacts with an alkene to form a cyclopropane. It is named after Howard Ensign Simmons, Jr. and Ronald D. Smith. It uses a methylene free radical intermediate that is delivered to both carbons of the alkene simultaneously, therefore the configuration of the double bond is preserved in the product and the reaction is stereospecific.

The Nicolaou Taxol total synthesis, published by K. C. Nicolaou and his group in 1994 concerns the total synthesis of taxol. Taxol is an important drug in the treatment of cancer but also expensive because the compound is harvested from a scarce resource, namely the pacific yew.

The McMurry reaction is an organic reaction in which two ketone or aldehyde groups are coupled to form an alkene using a titanium chloride compound such as titanium(III) chloride and a reducing agent. The reaction is named after its co-discoverer, John E. McMurry. The McMurry reaction originally involved the use of a mixture TiCl3 and LiAlH4, which produces the active reagents. Related species have been developed involving the combination of TiCl3 or TiCl4 with various other reducing agents, including potassium, zinc, and magnesium. This reaction is related to the Pinacol coupling reaction which also proceeds by reductive coupling of carbonyl compounds.

Chloroplatinic acid (also known as hexachloroplatinic acid) is an inorganic compound with the formula [H3O]2[PtCl6](H2O)x (0 ≤ x ≤ 6). A red solid, it is an important commercial source of platinum, usually as an aqueous solution. Although often written in shorthand as H2PtCl6, it is the hydronium (H3O+) salt of the hexachloroplatinate anion (PtCl2−
6). Hexachloroplatinic acid is highly hygroscopic.

Titanocene dichloride is the organotitanium compound with the formula (η5-C5H5)2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air. It shows antitumour activity and was the first non-platinum complex to undergo clinical trials as a chemotherapy drug.
This is the list of extremely hazardous substances defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act. The list can be found as an appendix to 40 CFR 355. Updates as of 2006 can be seen on the Federal Register, 71 FR 47121.

Borohydride refers to the anion [BH4]−, which is also called tetrahydridoborate, and its salts. Borohydride or hydroborate is also the term used for compounds containing [BH4−nXn]−, where n is an integer from 0 to 3, for example cyanoborohydride or cyanotrihydroborate [BH3(CN)]− and triethylborohydride or triethylhydroborate [BH(CH2CH3)3]−. Borohydrides find wide use as reducing agents in organic synthesis. The most important borohydrides are lithium borohydride and sodium borohydride, but other salts are well known. Tetrahydroborates are also of academic and industrial interest in inorganic chemistry.

Organozinc chemistry is the study of the physical properties, synthesis, and reactions of organozinc compounds, which are organometallic compounds that contain carbon (C) to zinc (Zn) chemical bonds.

A Rieke metal is a highly reactive metal powder generated by reduction of a metal salt with an alkali metal. These materials are named after Reuben D. Rieke, who first described along with an associate in 1972 the recipes for their preparation. In 1974 he told about Rieke-magnesium. A 1989 paper by Rieke lists several metals that are allowed by the periodic table to be produced by his process: Cd, Zn, Ni, Pt, Pd, Fe, In, Tl, Co, Cr, Mo, W, Cu, which in turn are called Rieke-nickel, Rieke-platinum, etc.
The reduction of nitro compounds are chemical reactions of wide interest in organic chemistry. The conversion can be effected by many reagents. The nitro group was one of the first functional groups to be reduced. Alkyl and aryl nitro compounds behave differently. Most useful is the reduction of aryl nitro compounds.

Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp, where Cp− is the cyclopentadienide anion. Sodium cyclopentadienide is a colorless solid, although samples often are pink owing to traces of oxidized impurities.
Organosodium chemistry is the chemistry of organometallic compounds containing a carbon to sodium chemical bond. The application of organosodium compounds in chemistry is limited in part due to competition from organolithium compounds, which are commercially available and exhibit more convenient reactivity.
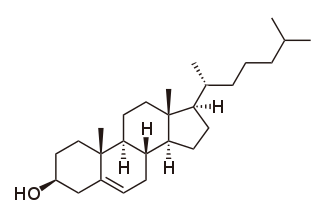
Cholesterol total synthesis in chemistry describes the total synthesis of the complex biomolecule cholesterol and is considered a great scientific achievement. The research group of Robert Robinson with John Cornforth published their synthesis in 1951 and that of Robert Burns Woodward with Franz Sondheimer in 1952. Both groups competed for the first publication since 1950 with Robinson having started in 1932 and Woodward in 1949. According to historian Greg Mulheirn the Robinson effort was hampered by his micromanagement style of leadership and the Woodward effort was greatly facilitated by his good relationships with chemical industry. Around 1949 steroids like cortisone were produced from natural resources but expensive. Chemical companies Merck & Co. and Monsanto saw commercial opportunities for steroid synthesis and not only funded Woodward but also provided him with large quantities of certain chemical intermediates from pilot plants. Hard work also helped the Woodward effort: one of the intermediate compounds was named Christmasterone as it was synthesized on Christmas Day 1950 by Sondheimer.
UV is the +5 oxidation state of uranium which is found in the form of [UO2]1+. This species is known as pentavalent uranyl cation and has a low stability due to the disproportionation into tetravalent and hexavalent uranium species.
In organometallic chemistry, metal–halogen exchange is a fundamental reaction that converts an organic halide into an organometallic product. The reaction commonly involves the use of electropositive metals and organochlorides, bromides, and iodides. Particularly well-developed is the use of metal–halogen exchange for the preparation of organolithium compounds.













