
In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

An acetate is a salt formed by the combination of acetic acid with a base. "Acetate" also describes the conjugate base or ion typically found in aqueous solution and written with the chemical formula C
2H
3O−
2. The neutral molecules formed by the combination of the acetate ion and a positive ion are also commonly called "acetates". The simplest of these is hydrogen acetate with corresponding salts, esters, and the polyatomic anion CH
3CO−
2, or CH
3COO−
.
The Brønsted–Lowry theory (also called proton theory of acids and bases) is an acid–base reaction theory which was proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923. The fundamental concept of this theory is that when an acid and a base react with each other, the acid forms its conjugate base, and the base forms its conjugate acid by exchange of a proton (the hydrogen cation, or H+). This theory is a generalization of the Arrhenius theory.

Organoboron chemistry or organoborane chemistry is the chemistry of organoboron compounds or organoboranes, which are chemical compounds of boron and carbon that are organic derivatives of borane (BH3), for example trialkyl boranes..

Zinc acetate is a salt with the formula Zn(CH3CO2)2, which commonly occurs as the dihydrate Zn(CH3CO2)2·2H2O. Both the hydrate and the anhydrous forms are colorless solids that are used as dietary supplements. When used as a food additive, it has the E number E650.
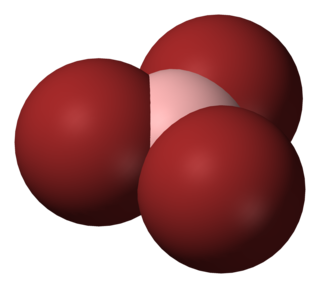
Boron tribromide, BBr3, is a colorless, fuming liquid compound containing boron and bromine. Commercial samples usually are amber to red/brown, due to weak bromine contamination. It is decomposed by water and alcohols.
Calcium magnesium acetate (CMA) is a deicer and can be used as an alternative to road salt. It is approximately as corrosive as normal tap water, and in varying concentrations can be effective in stopping road ice from forming down to around −27.5 °C (−17.5 °F) (its eutectic temperature). CMA can also be used as an H2S capture agent.
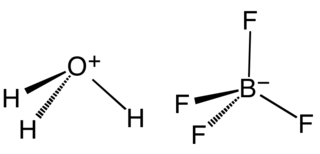
Fluoroboric acid or tetrafluoroboric acid is an inorganic compound with the simplified chemical formula H+[BF4]−. Unlike other strong acids like H2SO4 or HClO4, the pure tetrafluoroboric acid does not exist. The term "fluoroboric acid" refers to a range of chemical compounds, depending on the solvent. The H+ in the simplified formula of fluoroboric acid represents the solvated proton. The solvent can be any suitable Lewis base. For instance, if the solvent is water, fluoroboric acid can be represented by the formula [H3O]+[BF4]−, although more realistically, several water molecules solvate the proton: [H(H2O)n]+[BF4]−. The ethyl ether solvate is also commercially available, where the fluoroboric acid can be represented by the formula [H( 2O)n]+[BF4]−, where n is most likely 2.
Acetyl iodide is an organoiodine compound with the formula CH3COI. It is a colourless liquid. It is formally derived from acetic acid. Although far rarer in the laboratory than the related acetyl bromide and acetyl chloride, acetyl iodide is produced, transiently at least, on a far larger scale than any other acid halide. Specifically, it is generated by the carbonylation of methyl iodide in the Cativa and Monsanto processes, which are the main industrial processes that generate acetic acid. It is also an intermediate in the production of acetic anhydride from methyl acetate.

Cobalt(II) acetate is the cobalt salt of acetic acid. It is commonly found as the tetrahydrate Co(CH3CO2)2·4 H2O, abbreviated Co(OAc)2·4 H2O. It is used as a catalyst.

Acetic acid, systematically named ethanoic acid, is an acidic, colourless liquid and organic compound with the chemical formula CH3COOH. Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water and other trace elements.
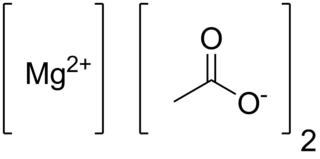
Anhydrous magnesium acetate has the chemical formula Mg(C2H3O2)2 and in its hydrated form, magnesium acetate tetrahydrate, it has the chemical formula Mg(CH3COO)2 • 4H2O. In this compound magnesium has an oxidation state of 2+. Magnesium acetate is the magnesium salt of acetic acid. It is deliquescent and upon heating, it decomposes to form magnesium oxide. Magnesium acetate is commonly used as a source of magnesium in biological reactions.

Barium acetate (Ba(C2H3O2)2) is the salt of barium(II) and acetic acid. Barium acetate is toxic to humans, but has use in chemistry and manufacturing.

Trimethylborane (TMB) is a toxic, pyrophoric gas with the formula B(CH3)3 (which can also be written as Me3B, with Me representing methyl).
An insertion reaction is a chemical reaction where one chemical entity interposes itself into an existing bond of typically a second chemical entity e.g.:

Nickel(II) acetate is the name for the coordination compounds with the formula Ni(CH3CO2)2·x H2O where x can be 0, 2, and 4. The green tetrahydrate Ni(CH3CO2)2·4 H2O is most common. It is used for electroplating.

Aluminium monoacetate, also known as dibasic aluminium acetate, and formally named dihydroxy aluminium acetate, is a salt of aluminium with acetic acid. It has the formula Al(OH)2(CH3COO), with aluminium in an oxidation state of +3, and appears under standard conditions as a white solid powder.

Neodymium acetate is an inorganic salt composed of a neodymium atom trication and three acetate groups as anions where neodymium exhibits the +3 oxidation state. It has a chemical formula of Nd(CH3COO)3 although it can be informally referred to as NdAc because Ac is an informal symbol for acetate. It commonly occurs as a light purple powder.
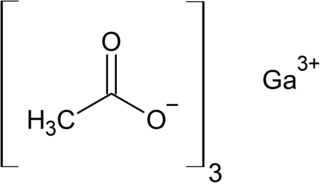
Gallium acetate is a salt composed of a gallium atom trication and three acetate groups as anions where gallium exhibits the +3 oxidation state. It has a chemical formula of Ga(CH3COO)3 although it can be informally referred to as GaAc because Ac is an informal symbol for acetate. Gallium is moderately water-soluble and decomposes to gallium oxide when heated to around 70 °C. Gallium acetate, like other acetate compounds, is a good precursor to ultra-pure compounds, catalysts and nanoscale materials. Gallium acetate is being considered as a substitute in de-icing compounds like calcium chloride and magnesium chloride.

Lutetium(III) acetate is the acetate salt of lutetium with the chemical formula of Lu(CH3COO)3.

















