
In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen atom with two electrons. The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are also called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. For inorganic chemists, hydrides refer to compounds and ions in which hydrogen is covalently attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed.

Boron hydride clusters are compounds with the formula BxHy or related anions, where x ≥ 3. Many such cluster compounds are known. Common examples are those with 5, 10, and 12 boron atoms. Although they have few practical applications, the borane hydride clusters exhibit structures and bonding that differs strongly from the patterns seen in hydrocarbons. Hybrids of boranes and hydrocarbons, the carboranes are also well developed.

Diborane(6), commonly known as diborane, is the chemical compound with the formula B2H6. It is a highly toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Given its simple formula, borane is a fundamental boron compound. It has attracted wide attention for its electronic structure. Several of its derivatives are useful reagents.

Decaborane, also called decaborane(14), is the inorganic compound with the chemical formula B10H14. It is classified as a borane and more specifically a boron hydride cluster. This white crystalline compound is one of the principal boron hydride clusters, both as a reference structure and as a precursor to other boron hydrides. It is toxic and volatile, giving off a foul odor, like that of burnt rubber or chocolate.

Pentaborane(9) is an inorganic compound with the formula B5H9. It is one of the most common boron hydride clusters, although it is a highly reactive compound. Because of its high reactivity with oxygen, it was once evaluated as rocket or jet fuel. Like many of the smaller boron hydrides, pentaborane is colourless, diamagnetic, and volatile. It is related to pentaborane(11).
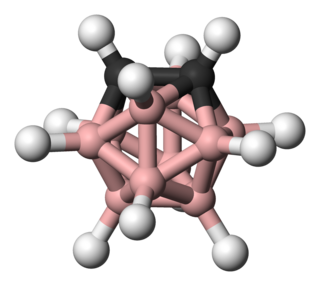
Carboranes are electron-delocalized clusters composed of boron, carbon and hydrogen atoms. Like many of the related boron hydrides, these clusters are polyhedra or fragments of polyhedra. Carboranes are one class of heteroboranes.

Hexaborane, also called hexaborane(10) to distinguish it from hexaborane(12) (B6H12), is a boron hydride cluster with the formula B6H10. It is a colorless liquid that is unstable in air.

Tetraborane was the first boron hydride compound to be discovered. It was classified by Alfred Stock and Carl Massenez in 1912 and was first isolated by Stock. It has a relatively low boiling point at 18 °C and is a gas at room temperature. Tetraborane gas is foul smelling and toxic.
Triethylborane (TEB), also called triethylboron, is an organoborane. It is a colorless pyrophoric liquid. Its chemical formula is (CH3CH2)3B or (C2H5)3B, abbreviated Et3B. It is soluble in organic solvents tetrahydrofuran and hexane.
Marion Frederick Hawthorne was an inorganic chemist who made contributions to the chemistry of boron hydrides, especially their clusters.
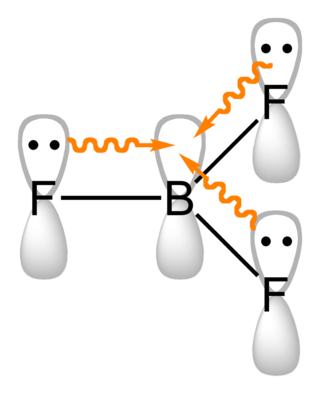
Boron compounds are compounds containing the element boron. In the most familiar compounds, boron has the formal oxidation state +3. These include oxides, sulfides, nitrides, and halides.
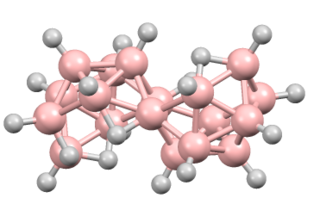
Octadecaborane is an inorganic compound, a boron hydride cluster with chemical formula B18H22. It is a colorless flammable solid, like many higher boron hydrides. Although the compound has no practical applications, its structure is of theoretical and pedagogical interest.

Caesium dodecaborate is an inorganic compound with the formula Cs2B12H12. It is a salt composed of caesium and dodecaborate(12) ions. The [B12H12]2− anion has been of great theoretical interest to the chemistry community.

Earl Muetterties, was an American inorganic chemist born in Illinois, who is known for his experimental work with boranes, homogeneous catalysis, heterogeneous catalysis, fluxional processes in organometallic complexes and apicophilicity.
Frederick Nye Tebbe was a chemist known for his work on organometallic chemistry. Tebbe was born in Oakland, California on March 20, 1935. His father, Charles L. Tebbe, worked for the United States Forest Service so Fred’s early education took place in Montana, Oregon, Maryland and Pennsylvania. He married Margaret Manzer in 1960, and they had a son and a daughter. He died of pancreatic cancer at his home in Delaware on September 28, 1995.
Borane, also known as borine, is an unstable and highly reactive molecule with the chemical formula BH
3. The preparation of borane carbonyl, BH3(CO), played an important role in exploring the chemistry of boranes, as it indicated the likely existence of the borane molecule. However, the molecular species BH3 is a very strong Lewis acid. Consequently, it is highly reactive and can only be observed directly as a continuously produced, transitory, product in a flow system or from the reaction of laser ablated atomic boron with hydrogen. It normally dimerizes to diborane in the absence of other chemicals.
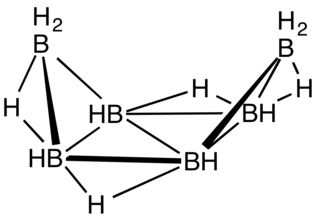
Hexaborane(12) is an inorganic compound with the formula B6H12. It is an obscure member of the boranes. It is a colorless liquid that, like some other boron hydride clusters, is readily hydrolyzed and flammable.

Octahydrotriborate is the boron hydride anion B3H8−. It forms a variety of salts that are colorless and air-stable. The tetrabutylammonium salt is soluble in organic solvents such as acetonitrile and methylene chloride. The anion is an intermediate is the synthesis of various higher boron hydrides, such as pentaborane(9). B3H8− can be viewed as the conjugate base of triborane B3H9.
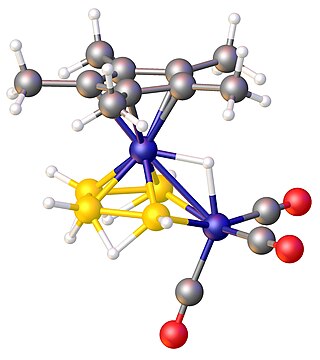
In chemistry, a metallaborane is a compound that contains one or more metal atoms and one or more boron hydride. These compounds are related conceptually and often synthetically to the boron-hydride clusters by replacement of BHn units with metal-containing fragments. Often these metal fragments are derived from metal carbonyls or cyclopentadienyl complexes. Their structures can often be rationalized by polyhedral skeletal electron pair theory. The inventory of these compounds is large, and their structures can be quite complex.

A borane is a compound with the formula BRxHy although examples include multi-boron derivatives. A large family of boron hydride clusters is also known. In addition to some applications in organic chemistry, the boranes have attracted much attention as they exhibit structures and bonding that differs strongly from the patterns seen in hydrocarbons. Hybrids of boranes and hydrocarbons, the carboranes, are also a well developed class of compounds.















