
Strontium is the chemical element with the symbol Sr and atomic number 38. An alkaline earth metal, strontium is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is exposed to air. Strontium has physical and chemical properties similar to those of its two vertical neighbors in the periodic table, calcium and barium. It occurs naturally mainly in the minerals celestine and strontianite, and is mostly mined from these.
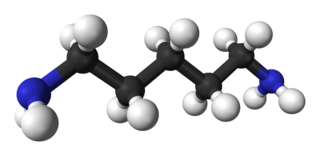
Cadaverine is an organic compound with the formula (CH2)5(NH2)2. Classified as diamine, it is a colorless liquid with an unpleasant odor. It is present in small quantities in living organisms but is often associated with the putrefaction of animal tissue.
In chemistry, a hydride is formally the anion of hydrogen (H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. For inorganic chemists, hydrides refer to compounds and ions in which hydrogen is covalently attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed.

Strontium chloride (SrCl2) is a salt of strontium and chloride. It is a 'typical' salt, forming neutral aqueous solutions. As with all compounds of strontium, this salt emits a bright red colour in flame, and is commonly used in fireworks to that effect. Its properties are intermediate between those for barium chloride, which is more toxic, and calcium chloride.
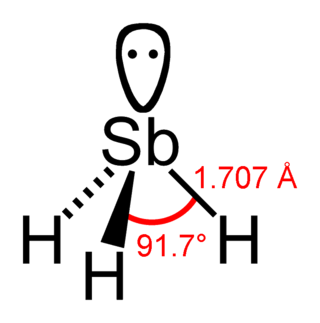
Stibine (IUPAC name: stibane) is a chemical compound with the formula SbH3. A pnictogen hydride, this colourless, highly toxic gas is the principal covalent hydride of antimony, and a heavy analogue of ammonia. The molecule is pyramidal with H–Sb–H angles of 91.7° and Sb–H distances of 170.7 pm (1.707 Å). This gas has an offensive smell like hydrogen sulfide (rotten eggs).

Germane is the chemical compound with the formula GeH4, and the germanium analogue of methane. It is the simplest germanium hydride and one of the most useful compounds of germanium. Like the related compounds silane and methane, germane is tetrahedral. It burns in air to produce GeO2 and water. Germane is a group 14 hydride.
Calcium hydride is the chemical compound with the formula CaH2, and is therefore an alkaline earth hydride. This grey powder reacts vigorously with water liberating hydrogen gas. CaH2 is thus used as a drying agent, i.e. a desiccant.
Polonium hydride (also known as polonium dihydride, hydrogen polonide, or polane) is a chemical compound with the formula PoH2. It is a liquid at room temperature, the second hydrogen chalcogenide with this property after water. It is very unstable chemically and tends to decompose into elemental polonium and hydrogen. It is a volatile and very labile compound, from which many polonides can be derived. Additionally, like all polonium compounds, it is highly radioactive.

Methylcyclopentane is an organic compound with the chemical formula CH3C5H9. It is a colourless, flammable liquid with a faint odor. It is a component of the naphthene fraction of petroleum. It usually is obtained as a mixture with cyclohexane. It is mainly converted in naphthene reformers to benzene. The C6 core of methylcyclopentane is not perfectly planar and can pucker to alleviate stress in its structure.
Binary compounds of hydrogen are binary chemical compounds containing just hydrogen and one other chemical element. By convention all binary hydrogen compounds are called hydrides even when the hydrogen atom in it is not an anion. These hydrogen compounds can be grouped into several types.
Chromium hydrides are compounds of chromium and hydrogen, and possibly other elements. Intermetallic compounds with not-quite-stoichometric quantities of hydrogen exist, as well as highly reactive molecules. When present at low concentrations, hydrogen and certain other elements alloyed with chromium act as softening agents that enables the movement of dislocations that otherwise not occur in the crystal lattices of chromium atoms.
Strontium chlorate is a chemical compound, with the formula Sr(ClO3)2. It is a strong oxidizing agent.
Iron(II) hydride, systematically named iron dihydride and poly(dihydridoiron) is solid inorganic compound with the chemical formula (FeH
2)
n (also written ([FeH
2])n or FeH
2). ). It is kinetically unstable at ambient temperature, and as such, little is known about its bulk properties. However, it is known as a black, amorphous powder, which was synthesised for the first time in 2014.
A polyhydride or superhydride is a compound that contains an abnormally large amount of hydrogen. This can be described as high hydrogen stoichiometry. Examples include iron pentahydride FeH5, LiH6, and LiH7. By contrast, the more well known lithium hydride only has one hydrogen atom.
Barium hydride is a chemical compound with the chemical formula BaH2.
An oxyhydride is a mixed anion compound containing both oxide O2− and hydride ions H−. These compounds may be unexpected as the hydrogen and oxygen could be expected to react to form water. But if the metals making up the cations are electropositive enough, and the conditions are reducing enough, solid materials can be made that combine hydrogen and oxygen in the negative ion role.
In chemistry, a hydridonitride is a chemical compound that contains hydride and nitride ions in a single phase. These inorganic compounds are distinct from inorganic amides and imides as the hydrogen does not share a bond with nitrogen, and contain a larger proportion of metals.
The inorganic imides are compounds containing an ion composed of nitrogen bonded to hydrogen with formula HN2−. Organic imides have the NH group, and two single or one double covalent bond to other atoms. The imides are related to the inorganic amides (H2N−), the nitrides (N3−) and the nitridohydrides (N3−•H−).
A silicide hydride is a mixed anion compound that contains silicide (Si4− or clusters) and hydride (H−) anions. The hydrogen is not bound to silicon in these compounds. These can be classed as interstitial hydrides, Hydrogenated zintl phases, or Zintl phase hydrides. In the related silanides, SiH3− anions or groups occur. Where hydrogen is bonded to the silicon, this is a case of anionic hydride, and where it is bonded to a more complex anion, it would be termed polyanionic hydride.








