In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and more frequently in the past) been applied to all compounds containing covalently bound H atoms. In this broad and potentially archaic sense, water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. In covalent compounds, it implies hydrogen is attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed.
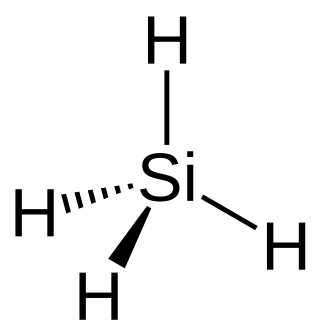
Silane (Silicane) is an inorganic compound with chemical formula SiH4. It is a colorless, pyrophoric, toxic gas with a sharp, repulsive, pungent smell, somewhat similar to that of acetic acid. Silane is of practical interest as a precursor to elemental silicon. Silane with alkyl groups are effective water repellents for mineral surfaces such as concrete and masonry. Silanes with both organic and inorganic attachments are used as coupling agents. They are commonly used to apply coatings to surfaces or as an adhesion promoter.

Arsine (IUPAC name: arsane) is an inorganic compound with the formula AsH3. This flammable, pyrophoric, and highly toxic pnictogen hydride gas is one of the simplest compounds of arsenic. Despite its lethality, it finds some applications in the semiconductor industry and for the synthesis of organoarsenic compounds. The term arsine is commonly used to describe a class of organoarsenic compounds of the formula AsH3−xRx, where R = aryl or alkyl. For example, As(C6H5)3, called triphenylarsine, is referred to as "an arsine".

Diborane(6), commonly known as diborane, is the chemical compound with the formula B2H6. It is a highly toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Given its simple formula, borane is a fundamental boron compound. It has attracted wide attention for its electronic structure. Several of its derivatives are useful reagents.

Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li[AlH4] or LiAlH4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides. The solid is dangerously reactive toward water, releasing gaseous hydrogen (H2). Some related derivatives have been discussed for hydrogen storage.

Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula NaBH4. It is a white crystalline solid, usually encountered as an aqueous basic solution. Sodium borohydride is a reducing agent that finds application in papermaking and dye industries. It is also used as a reagent in organic synthesis.
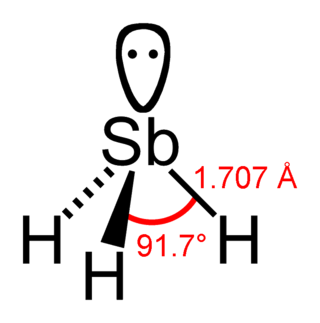
Stibine (IUPAC name: stibane) is a chemical compound with the formula SbH3. A pnictogen hydride, this colourless, highly toxic gas is the principal covalent hydride of antimony, and a heavy analogue of ammonia. The molecule is pyramidal with H–Sb–H angles of 91.7° and Sb–H distances of 170.7 pm (1.707 Å). The smell of this compound from usual sources (like from reduction of antimony compounds) is reminiscent of arsine, i.e. garlic-like.

Lithium hydride is an inorganic compound with the formula LiH. This alkali metal hydride is a colorless solid, although commercial samples are grey. Characteristic of a salt-like (ionic) hydride, it has a high melting point, and it is not soluble but reactive with all protic organic solvents. It is soluble and nonreactive with certain molten salts such as lithium fluoride, lithium borohydride, and sodium hydride. With a molar mass of 7.95 g/mol, it is the lightest ionic compound.

Aluminium hydride is an inorganic compound with the formula AlH3. Alane and its derivatives are part of a family of common reducing reagents in organic synthesis based around group 13 hydrides. In solution—typically in ethereal solvents such tetrahydrofuran or diethyl ether—aluminium hydride forms complexes with Lewis bases, and reacts selectively with particular organic functional groups, and although it is not a reagent of choice, it can react with carbon-carbon multiple bonds. Given its density, and with hydrogen content on the order of 10% by weight, some forms of alane are, as of 2016, active candidates for storing hydrogen and so for power generation in fuel cell applications, including electric vehicles. As of 2006 it was noted that further research was required to identify an efficient, economical way to reverse the process, regenerating alane from spent aluminium product.

Digallane is an inorganic compound with the chemical formula GaH2(H)2GaH2. It is the dimer of the monomeric compound gallane. The eventual preparation of the pure compound, reported in 1989, was hailed as a "tour de force." Digallane had been reported as early as 1941 by Wiberg; however, this claim could not be verified by later work by Greenwood and others. This compound is a colorless gas that decomposes above 0 °C.
Zinc hydride is an inorganic compound with the chemical formula ZnH2. It is a white, odourless solid which slowly decomposes into its elements at room temperature; despite this it is the most stable of the binary first row transition metal hydrides. A variety of coordination compounds containing Zn–H bonds are used as reducing agents, but ZnH2 itself has no common applications.

In organic chemistry, carbonyl reduction is the conversion of any carbonyl group, usually to an alcohol. It is a common transformation that is practiced in many ways. Ketones, aldehydes, carboxylic acids, esters, amides, and acid halides - some of the most pervasive functional groups, -comprise carbonyl compounds. Carboxylic acids, esters, and acid halides can be reduced to either aldehydes or a step further to primary alcohols, depending on the strength of the reducing agent. Aldehydes and ketones can be reduced respectively to primary and secondary alcohols. In deoxygenation, the alcohol group can be further reduced and removed altogether by replacement with H.
Binary compounds of hydrogen are binary chemical compounds containing just hydrogen and one other chemical element. By convention all binary hydrogen compounds are called hydrides even when the hydrogen atom in it is not an anion. These hydrogen compounds can be grouped into several types.
Cadmium hydride is an inorganic compound with the chemical formula (CdH
2)
n. It is a solid, known only as a thermally unstable, insoluble white powder.

Indium trihydride is an inorganic compound with the chemical formula. It has been observed in matrix isolation and laser ablation experiments. Gas phase stability has been predicted. The infrared spectrum was obtained in the gas phase by laser ablation of indium in presence of hydrogen gas InH3 is of no practical importance.

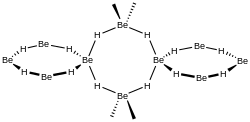
Beryllium borohydride is an inorganic compound with the chemical formula Be[BH4]2.

Copper hydride is an inorganic compound with the chemical formula CuHn where n ~ 0.95. It is a red solid, rarely isolated as a pure composition, that decomposes to the elements. Copper hydride is mainly produced as a reducing agent in organic synthesis and as a precursor to various catalysts.

1,2-Dimethyldiborane is an organoboron compound with the formula [(CH3)BH2]2. Structurally, it is related to diborane, but with methyl groups replacing terminal hydrides on each boron. It is the dimer of methylborane, CH3BH2, the simplest alkylborane. 1,2-Dimethyldiborane can exist in a cis- and a trans arrangement. 1,2-Dimethyldiborane is an easily condensed, colorless gas that ignites spontaneously in air.

Lithium tetrahydridogallate is the inorganic compound with formula LiGaH4. It is a white solid similar to but less thermally robust than lithium aluminium hydride.
Hydrogen compounds are compounds containing the element hydrogen. In these compounds, hydrogen can form in the +1 and -1 oxidation states. Hydrogen can form compounds both ionically and in covalent substances. It is a part of many organic compounds such as hydrocarbons as well as water and other organic substances. The H+ ion is often called a proton because it has one proton and no electrons, although the proton does not move freely. Brønsted–Lowry acids are capable of donating H+ ions to bases.