
Boron hydride clusters are compounds with the formula BxHy or related anions, where x ≥ 3. Many such cluster compounds are known. Common examples are those with 5, 10, and 12 boron atoms. Although they have few practical applications, the borane hydride clusters exhibit structures and bonding that differs strongly from the patterns seen in hydrocarbons. Hybrids of boranes and hydrocarbons, the carboranes are also well developed.

Diborane(6), commonly known as diborane, is the chemical compound with the formula B2H6. It is a highly toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Given its simple formula, borane is a fundamental boron compound. It has attracted wide attention for its electronic structure. Several of its derivatives are useful reagents.

Decaborane, also called decaborane(14), is the inorganic compound with the chemical formula B10H14. It is classified as a borane and more specifically a boron hydride cluster. This white crystalline compound is one of the principal boron hydride clusters, both as a reference structure and as a precursor to other boron hydrides. It is toxic and volatile, giving off a foul odor, like that of burnt rubber or chocolate.
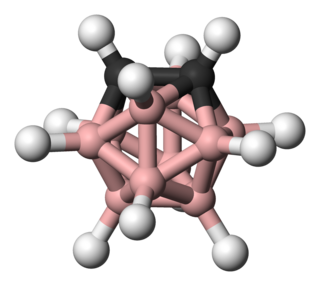
Carboranes are electron-delocalized clusters composed of boron, carbon and hydrogen atoms. Like many of the related boron hydrides, these clusters are polyhedra or fragments of polyhedra. Carboranes are one class of heteroboranes.

Hexaborane, also called hexaborane(10) to distinguish it from hexaborane(12) (B6H12), is a boron hydride cluster with the formula B6H10. It is a colorless liquid that is unstable in air.

Boron arsenide is a chemical compound involving boron and arsenic, usually with a chemical formula BAs. Other boron arsenide compounds are known, such as the subarsenide B12As2. Chemical synthesis of cubic BAs is very challenging and its single crystal forms usually have defects.
Lithium metaborate is a chemical compound of lithium, boron, and oxygen with elemental formula LiBO2. It is often encountered as a hydrate, LiBO2·nH2O, where n is usually 2 or 4. However, these formulas do not describe the actual structure of the solids.
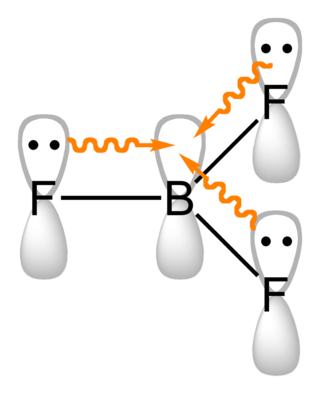
Boron compounds are compounds containing the element boron. In the most familiar compounds, boron has the formal oxidation state +3. These include oxides, sulfides, nitrides, and halides.

Lithium tetrafluoroborate is an inorganic compound with the formula LiBF4. It is a white crystalline powder. It has been extensively tested for use in commercial secondary batteries, an application that exploits its high solubility in nonpolar solvents.
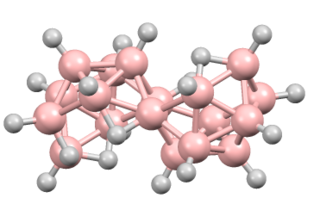
Octadecaborane is an inorganic compound, a boron hydride cluster with chemical formula B18H22. It is a colorless flammable solid, like many higher boron hydrides. Although the compound has no practical applications, its structure is of theoretical and pedagogical interest.
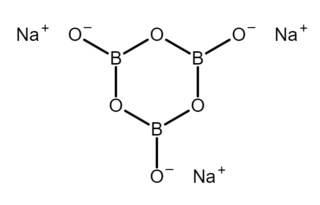
Sodium metaborate is a chemical compound of sodium, boron, and oxygen with formula NaBO2. However, the metaborate ion is trimeric in the anhydrous solid, therefore a more correct formula is Na3B3O6 or (Na+)3[B3O6]3−. The formula can be written also as Na2O·B2O3 to highlight the relation to the main oxides of sodium and boron. The name is also applied to several hydrates whose formulas can be written NaBO2·nH2O for various values of n.
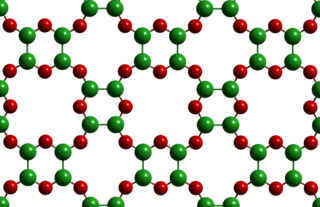
Boron monoxide (BO) is a binary compound of boron and oxygen. It has a molar mass of 26.81 g/mol. The material was first reported in 1940, with a modified synthetic procedure published in 1955, however, the material's structure had remained unknown for nearly a century. A number of allotropes of BO have been theorized ranging from molecular species, to 1D, 2D, and 3D-structured materials, but these were difficult to differentiate using common structural characterization methods. Recent work suggests that the material forms 2D nanosheets composed of O-bridged B4O2 rings, a structure initially postulated in 1961. Due to the lack of precise structural information on the identity of the compound, it has not found widespread use in industry.
Boron monofluoride or fluoroborylene is a chemical compound with the formula BF, one atom of boron and one of fluorine. It is an unstable gas, but it is a stable ligand on transition metals, in the same way as carbon monoxide. It is a subhalide, containing fewer than the normal number of fluorine atoms, compared with boron trifluoride. It can also be called a borylene, as it contains boron with two unshared electrons. BF is isoelectronic with carbon monoxide and dinitrogen; each molecule has 14 electrons.

Eluvathingal Devassy Jemmis is a professor of theoretical chemistry at the Indian Institute of Science, Bangalore, India. He was the founding director of Indian Institute of Science Education and Research, Thiruvananthapuram (IISER-TVM). His primary area of research is applied theoretical chemistry with emphasis on structure, bonding and reactivity, across the periodic table of the elements. Apart from many of his contributions to applied theoretical chemistry, an equivalent of the structural chemistry of carbon, as exemplified by the Huckel 4n+2 Rule, benzenoid aromatics and graphite, and tetrahedral carbon and diamond, is brought in the structural chemistry of boron by the Jemmis mno rules which relates polyhedral and macropolyhedral boranes to allotropes of boron and boron-rich solids. He has been awarded Padma Shri in Science and Engineering category by the Government of India.

Lai-Sheng Wang is an experimental physical chemist currently serving as the Chair of the Chemistry Department at Brown University. Wang is known for his work on atomic gold pyramids and planar boron clusters.
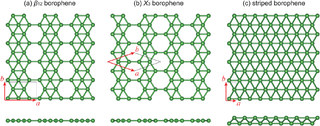
Borophene is a crystalline atomic monolayer of boron, i.e., it is a two-dimensional allotrope of boron and also known as boron sheet. First predicted by theory in the mid-1990s, different borophene structures were experimentally confirmed in 2015.
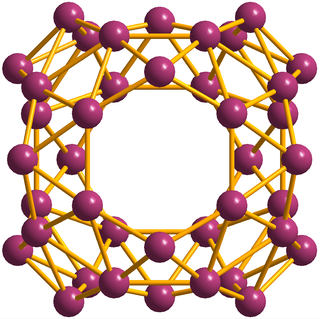
Borospherene (B40) is an electron-deficient cluster molecule containing 40 boron atoms. It bears similarities to other homoatomic cluster structures such as buckminsterfullerene (C60), stannaspherene, and plumbaspherene, but with a different symmetry. The first experimental evidence for borospherene was reported in July 2014, and is described in the journal Nature Chemistry. The molecule includes unusual hexagonal and heptagonal faces. Despite many calculation-based investigations into its structure and properties, a viable route for the synthesis and isolation of borospherene has yet to be established, and as a consequence it is still relatively poorly understood.

Pentaborane(11) is inorganic compound with the chemical formula B5H11. It is an obscure boron hydride cluster, especially relative to the heavily studied pentaborane(9) (B5H9). With two more hydrogen atoms than nido-pentaborane(9), pentaborane(11) is classified as an arachno- cluster.
Group 13 hydrides are chemical compounds containing group 13-hydrogen bonds.

ortho-Carborane is the organoboron compound with the formula C2B10H12. The prefix ortho is derived from ortho. It is the most prominent carborane. This derivative has been considered for a wide range of applications from heat-resistant polymers to medical applications. It is a colorless solid that melts, without decomposition, at 320 °C
















