
Europium is a chemical element; it has symbol Eu and atomic number 63. Europium is a silvery-white metal of the lanthanide series that reacts readily with air to form a dark oxide coating. It is the most chemically reactive, least dense, and softest of the lanthanide elements. It is soft enough to be cut with a knife. Europium was isolated in 1901 and named after the continent of Europe. Europium usually assumes the oxidation state +3, like other members of the lanthanide series, but compounds having oxidation state +2 are also common. All europium compounds with oxidation state +2 are slightly reducing. Europium has no significant biological role and is relatively non-toxic compared to other heavy metals. Most applications of europium exploit the phosphorescence of europium compounds. Europium is one of the rarest of the rare-earth elements on Earth.

Hydrogen is a chemical element; it has symbol H and atomic number 1. It is the lightest element and, at standard conditions, is a gas of diatomic molecules with the formula H2, sometimes called dihydrogen, but more commonly called hydrogen gas, molecular hydrogen or simply hydrogen. It is colorless, odorless, non-toxic, and highly combustible. Constituting about 75% of all normal matter, hydrogen is the most abundant chemical element in the universe. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found in water, organic compounds, as dihydrogen, and in other molecular forms. The most common isotope of hydrogen consists of one proton, one electron, and no neutrons.
The lanthanide or lanthanoid series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium is also sometimes considered a lanthanide, despite being a d-block element and a transition metal.

In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and more frequently in the past) been applied to all compounds containing covalently bound H atoms. In this broad and potentially archaic sense, water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. In covalent compounds, it implies hydrogen is attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed.
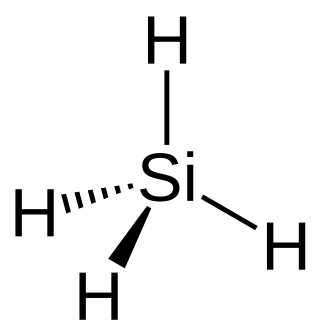
Silane (Silicane) is an inorganic compound with chemical formula SiH4. It is a colorless, pyrophoric, toxic gas with a sharp, repulsive, pungent smell, somewhat similar to that of acetic acid. Silane is of practical interest as a precursor to elemental silicon. Silane with alkyl groups are effective water repellents for mineral surfaces such as concrete and masonry. Silanes with both organic and inorganic attachments are used as coupling agents. They are commonly used to apply coatings to surfaces or as an adhesion promoter.

Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons.
Calcium hydride is the chemical compound with the formula CaH2, an alkaline earth hydride. This grey powder reacts vigorously with water, liberating hydrogen gas. CaH2 is thus used as a drying agent, i.e. a desiccant.

Magnesium hydride is the chemical compound with the molecular formula MgH2. It contains 7.66% by weight of hydrogen and has been studied as a potential hydrogen storage medium.
Binary compounds of hydrogen are binary chemical compounds containing just hydrogen and one other chemical element. By convention all binary hydrogen compounds are called hydrides even when the hydrogen atom in it is not an anion. These hydrogen compounds can be grouped into several types.
An yttrium compound is a chemical compound containing yttrium. Among these compounds, yttrium generally has a +3 valence. The solubility properties of yttrium compounds are similar to those of the lanthanides. For example oxalates and carbonates are hardly soluble in water, but soluble in excess oxalate or carbonate solutions as complexes are formed. Sulfates and double sulfates are generally soluble. They resemble the "yttrium group" of heavy lanthanide elements.
An oxyhydride is a mixed anion compound containing both oxide O2− and hydride ions H−. These compounds may be unexpected as the hydrogen and oxygen could be expected to react to form water. But if the metals making up the cations are electropositive enough, and the conditions are reducing enough, solid materials can be made that combine hydrogen and oxygen in the negative ion role.
The inorganic imide is an inorganic chemical compound containing

Europium(II) chloride is an inorganic compound with a chemical formula EuCl2. When it is irradiated by ultraviolet light, it has bright blue fluorescence.
Ytterbium(II) hydride is the hydride of ytterbium with the chemical formula YbH2. In this compound, the ytterbium atom has an oxidation state of +2 and the hydrogen atoms have an oxidation state of -1. Its resistivity at room temperature is 107 Ω·cm. Ytterbium hydride has a high thermostability.
An arsinide, arsanide, dihydridoarsenate(1−) or arsanyl compound is a chemical derivative of arsine, where one hydrogen atom is replaced with a metal or cation. The arsinide ion has formula AsH−2. It can be considered as a ligand with name arsenido or arsanido. Few chemists study arsanyl compounds, as they are both toxic and unstable. The IUPAC names are arsanide and dihydridoarsenate(1−). For the ligand the name is arsanido. The neutral −AsH2 group is termed arsanyl.

Europium(II) oxide (EuO) is a chemical compound which is one of the oxides of europium. In addition to europium(II) oxide, there is also europium(III) oxide and the mixed valence europium(II,III) oxide.

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Many europium compounds fluoresce under ultraviolet light due to the excitation of electrons to higher energy levels. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.
Europium(III) oxalate (Eu2(C2O4)3) is a chemical compound of europium and oxalic acid. There are different hydrates including the decahydrate, hexahydrate and tetrahydrate. Europium(II) oxalate is also known.
Hydrogen compounds are compounds containing the element hydrogen. In these compounds, hydrogen can form in the +1 and -1 oxidation states. Hydrogen can form compounds both ionically and in covalent substances. It is a part of many organic compounds such as hydrocarbons as well as water and other organic substances. The H+ ion is often called a proton because it has one proton and no electrons, although the proton does not move freely. Brønsted–Lowry acids are capable of donating H+ ions to bases.
Lanthanide compounds are compounds formed by the 15 elements classed as lanthanides. The lanthanides are generally trivalent, although some, such as cerium and europium, are capable of forming compounds in other oxidation states.










