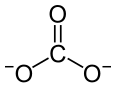
A carbonate is a salt of carbonic acid,, characterized by the presence of the carbonate ion, a polyatomic ion with the formula CO2−3. The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate groupO=C(−O−)2.

Calcium carbonate is a chemical compound with the chemical formula CaCO3. It is a common substance found in rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skeletons and pearls. Materials containing much calcium carbonate or resembling it are described as calcareous. Calcium carbonate is the active ingredient in agricultural lime and is produced when calcium ions in hard water react with carbonate ions to form limescale. It has medical use as a calcium supplement or as an antacid, but excessive consumption can be hazardous and cause hypercalcemia and digestive issues.

Sodium carbonate is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water. Historically, it was extracted from the ashes of plants grown in sodium-rich soils, and because the ashes of these sodium-rich plants were noticeably different from ashes of wood, sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process, as well as by carbonating sodium hydroxide which is made using the chloralkali process.
Carbonation is the chemical reaction of carbon dioxide to give carbonates, bicarbonates, and carbonic acid. In chemistry, the term is sometimes used in place of carboxylation, which refers to the formation of carboxylic acids.

Magnesium carbonate, MgCO3, is an inorganic salt that is a colourless or white solid. Several hydrated and basic forms of magnesium carbonate also exist as minerals.

Ammonium bicarbonate is an inorganic compound with formula (NH4)HCO3. The compound has many names, reflecting its long history. Chemically speaking, it is the bicarbonate salt of the ammonium ion. It is a colourless solid that degrades readily to carbon dioxide, water and ammonia.

Praseodymium(III) chloride is the inorganic compound with the formula PrCl3. Like other lanthanide trichlorides, it exists both in the anhydrous and hydrated forms. It is a blue-green solid that rapidly absorbs water on exposure to moist air to form a light green heptahydrate.

Nickel(II) carbonate describes one or a mixture of inorganic compounds containing nickel and carbonate. From the industrial perspective, an important nickel carbonate is basic nickel carbonate with the formula Ni4CO3(OH)6(H2O)4. Simpler carbonates, ones more likely encountered in the laboratory, are NiCO3 and its hexahydrate. All are paramagnetic green solids containing Ni2+ cations. The basic carbonate is an intermediate in the hydrometallurgical purification of nickel from its ores and is used in electroplating of nickel.
Carbamic acid, which might also be called aminoformic acid or aminocarboxylic acid, is the chemical compound with the formula H2NCOOH. It can be obtained by the reaction of ammonia NH3 and carbon dioxide CO2 at very low temperatures, which also yields ammonium carbamate [NH4]+[NH2CO2]−. The compound is stable only up to about 250 K (−23 °C); at higher temperatures it decomposes into those two gases. The solid apparently consists of dimers, with the two molecules connected by hydrogen bonds between the two carboxyl groups –COOH.

Strontium carbonate (SrCO3) is the carbonate salt of strontium that has the appearance of a white or grey powder. It occurs in nature as the mineral strontianite.

Barium chlorate, Ba(ClO3)2, is the barium salt of chloric acid. It is a white crystalline solid, and like all soluble barium compounds, irritant and toxic. It is sometimes used in pyrotechnics to produce a green colour. It also finds use in the production of chloric acid.

Terbium(III) nitrate is an inorganic chemical compound, a salt of terbium and nitric acid, with the formula Tb(NO3)3. The hexahydrate crystallizes as triclinic colorless crystals with the formula [Tb(NO3)3(H2O)4]·2H2O. It can be used to synthesize materials with green emission.

A carbonate fluoride, fluoride carbonate, fluorocarbonate or fluocarbonate is a double salt containing both carbonate and fluoride. The salts are usually insoluble in water, and can have more than one kind of metal cation to make more complex compounds. Rare-earth fluorocarbonates are particularly important as ore minerals for the light rare-earth elements lanthanum, cerium and neodymium. Bastnäsite is the most important source of these elements. Other artificial compounds are under investigation as non-linear optical materials and for transparency in the ultraviolet, with effects over a dozen times greater than Potassium dideuterium phosphate.

Total inorganic carbon is the sum of the inorganic carbon species.
The carbonate oxalates are mixed anion compounds that contain both carbonate (CO3) and oxalate (C2O4) anions. Most compounds incorporate large trivalent metal ions, such as the rare earth elements. Some carbonate oxalate compounds of variable composition are formed by heating oxalates.

Neodymium compounds are compounds formed by the lanthanide metal neodymium (Nd). In these compounds, neodymium generally exhibits the +3 oxidation state, such as NdCl3, Nd2(SO4)3 and Nd(CH3COO)3. Compounds with neodymium in the +2 oxidation state are also known, such as NdCl2 and NdI2. Some neodymium compounds have colors that vary based upon the type of lighting.
Neodymium(III) carbonate is an inorganic compound, a salt, where neodymium is in the +3 oxidation state and the carbonate ion has charge -2. It has a chemical formula of Nd2(CO3)3. The anhydrous form is purple-red, while the octahydrate is a pink solid. Both of these salts are insoluble in water.

Europium(III) acetate is an inorganic salt of europium and acetic acid with the chemical formula of Eu(CH3COO)3. In this compound, europium exhibits the +3 oxidation state. It can exist in the anhydrous form, sesquihydrate and tetrahydrate. Its hydrate molecule is a dimer.

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Many europium compounds fluoresce under ultraviolet light due to the excitation of electrons to higher energy levels. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.
Europium(III) oxalate (Eu2(C2O4)3) is a chemical compound of europium and oxalic acid. There are different hydrates including the decahydrate, hexahydrate and tetrahydrate. Europium(II) oxalate is also known.