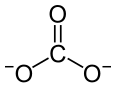
A carbonate is a salt of carbonic acid, H2CO3, characterized by the presence of the carbonate ion, a polyatomic ion with the formula CO2−3. The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate groupO=C(−O−)2.

Sodium carbonate is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water. Historically, it was extracted from the ashes of plants grown in sodium-rich soils, and because the ashes of these sodium-rich plants were noticeably different from ashes of wood, sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process, as well as by carbonating sodium hydroxide which is made using the Chlor-alkali process.

Magnesium carbonate, MgCO3, is an inorganic salt that is a colourless or white solid. Several hydrated and basic forms of magnesium carbonate also exist as minerals.

Barium hydroxide is a chemical compound with the chemical formula Ba(OH)2. The monohydrate (x = 1), known as baryta or baryta-water, is one of the principal compounds of barium. This white granular monohydrate is the usual commercial form.

Barium carbonate is the inorganic compound with the formula BaCO3. Like most alkaline earth metal carbonates, it is a white salt that is poorly soluble in water. It occurs as the mineral known as witherite. In a commercial sense, it is one of the most important barium compounds.

Praseodymium(III) chloride is the inorganic compound with the formula PrCl3. Like other lanthanide trichlorides, it exists both in the anhydrous and hydrated forms. It is a blue-green solid that rapidly absorbs water on exposure to moist air to form a light green heptahydrate.
Neodymium(III) chloride or neodymium trichloride is a chemical compound of neodymium and chlorine with the formula NdCl3. This anhydrous compound is a mauve-colored solid that rapidly absorbs water on exposure to air to form a purple-colored hexahydrate, NdCl3·6H2O. Neodymium(III) chloride is produced from minerals monazite and bastnäsite using a complex multistage extraction process. The chloride has several important applications as an intermediate chemical for production of neodymium metal and neodymium-based lasers and optical fibers. Other applications include a catalyst in organic synthesis and in decomposition of waste water contamination, corrosion protection of aluminium and its alloys, and fluorescent labeling of organic molecules (DNA).
A solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and anions dissolve in or precipitate from solution.

Hexafluorosilicic acid is an inorganic compound with the chemical formula H
2SiF
6. Aqueous solutions of hexafluorosilicic acid consist of salts of the cation and hexafluorosilicate anion. These salts and their aqueous solutions are colorless.

Nickel(II) carbonate describes one or a mixture of inorganic compounds containing nickel and carbonate. From the industrial perspective, an important nickel carbonate is basic nickel carbonate with the formula Ni4CO3(OH)6(H2O)4. Simpler carbonates, ones more likely encountered in the laboratory, are NiCO3 and its hexahydrate. All are paramagnetic green solids containing Ni2+ cations. The basic carbonate is an intermediate in the hydrometallurgical purification of nickel from its ores and is used in electroplating of nickel.
Uranium compounds are compounds formed by the element uranium (U). Although uranium is a radioactive actinide, its compounds are well studied due to its long half-life and its applications. It usually forms in the +4 and +6 oxidation states, although it can also form in other oxidation states.

Strontium carbonate (SrCO3) is the carbonate salt of strontium that has the appearance of a white or grey powder. It occurs in nature as the mineral strontianite.

Barium chlorate, Ba(ClO3)2, is the barium salt of chloric acid. It is a white crystalline solid, and like all soluble barium compounds, irritant and toxic. It is sometimes used in pyrotechnics to produce a green color. It also finds use in the production of chloric acid.

Compounds of lead exist with lead in two main oxidation states: +2 and +4. The former is more common. Inorganic lead(IV) compounds are typically strong oxidants or exist only in highly acidic solutions.

Neodymium(III) fluoride is an inorganic chemical compound of neodymium and fluorine with the formula NdF3. It is a purplish pink colored solid with a high melting point.

Neodymium(III) acetate is an inorganic salt composed of a neodymium atom trication and three acetate groups as anions where neodymium exhibits the +3 oxidation state. It has a chemical formula of Nd(CH3COO)3 although it can be informally referred to as NdAc because Ac is an informal symbol for acetate. It commonly occurs as a light purple powder.

Neodymium compounds are compounds formed by the lanthanide metal neodymium (Nd). In these compounds, neodymium generally exhibits the +3 oxidation state, such as NdCl3, Nd2(SO4)3 and Nd(CH3COO)3. Compounds with neodymium in the +2 oxidation state are also known, such as NdCl2 and NdI2. Some neodymium compounds have colors that vary based upon the type of lighting.
Praseodymium(III) carbonate is an inorganic compound, with a chemical formula of Pr2(CO3)3. The anhydrous form is olive green, and many of its hydrates such as heptahydrate and octahydrate are known. They are all insoluble in water.
Cobalt compounds are chemical compounds formed by cobalt with other elements.
Europium(III) carbonate is an inorganic compound with the chemical formula Eu2(CO3)3.