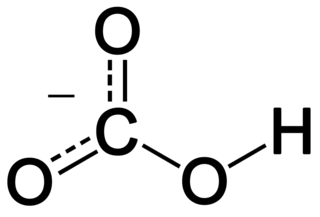
In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula HCO−
3.

Calcium carbonate is a chemical compound with the chemical formula CaCO3. It is a common substance found in rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skeletons and pearls. Materials containing much calcium carbonate or resembling it are described as calcareous. Calcium carbonate is the active ingredient in agricultural lime and is produced when calcium ions in hard water react with carbonate ions to form limescale. It has medical use as a calcium supplement or as an antacid, but excessive consumption can be hazardous and cause hypercalcemia and digestive issues.

Weathering is the deterioration of rocks, soils and minerals through contact with water, atmospheric gases, sunlight, and biological organisms. It occurs in situ, and so is distinct from erosion, which involves the transport of rocks and minerals by agents such as water, ice, snow, wind, waves and gravity.

Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation (Na+) and a bicarbonate anion (HCO3−). Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda (sodium carbonate). The natural mineral form is nahcolite, although it is more commonly found as a component of the mineral trona.

Sodium carbonate is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water. Historically, it was extracted from the ashes of plants grown in sodium-rich soils, and because the ashes of these sodium-rich plants were noticeably different from ashes of wood, sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process, as well as by carbonating sodium hydroxide which is made using the chloralkali process.
Carbonation is the chemical reaction of carbon dioxide to give carbonates, bicarbonates, and carbonic acid. In chemistry, the term is sometimes used in place of carboxylation, which refers to the formation of carboxylic acids.

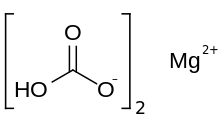
Magnesium carbonate, MgCO3, is an inorganic salt that is a colourless or white solid. Several hydrated and basic forms of magnesium carbonate also exist as minerals.

Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca(OH)2. It is a colorless crystal or white powder and is produced when quicklime (calcium oxide) is mixed with water. Annually, approximately 125 million tons of calcium hydroxide are produced worldwide.

Soda lime, a mixture of sodium hydroxide (NaOH) and calcium oxide (CaO), is used in granular form within recirculating breathing environments like general anesthesia and its breathing circuit, submarines, rebreathers, and hyperbaric chambers and underwater habitats. Its purpose is to eliminate carbon dioxide from breathing gases, preventing carbon dioxide retention and, eventually, carbon dioxide poisoning. The creation of soda lime involves treating slaked lime with a concentrated sodium hydroxide solution.
The Solvay process or ammonia–soda process is the major industrial process for the production of sodium carbonate (soda ash, Na2CO3). The ammonia–soda process was developed into its modern form by the Belgian chemist Ernest Solvay during the 1860s. The ingredients for this are readily available and inexpensive: salt brine (from inland sources or from the sea) and limestone (from quarries). The worldwide production of soda ash in 2005 was estimated at 42 million tonnes, which is more than six kilograms (13 lb) per year for each person on Earth. Solvay-based chemical plants now produce roughly three-quarters of this supply, with the remaining being mined from natural deposits. This method superseded the Leblanc process.
An acidic oxide is an oxide that either produces an acidic solution upon addition to water, or acts as an acceptor of hydroxide ions effectively functioning as a Lewis acid. Acidic oxides will typically have a low pKa and may be inorganic or organic. A commonly encountered acidic oxide, carbon dioxide produces an acidic solution when dissolved.

Water softening is the removal of calcium, magnesium, and certain other metal cations in hard water. The resulting soft water requires less soap for the same cleaning effort, as soap is not wasted bonding with calcium ions. Soft water also extends the lifetime of plumbing by reducing or eliminating scale build-up in pipes and fittings. Water softening is usually achieved using lime softening or ion-exchange resins, but is increasingly being accomplished using nanofiltration or reverse osmosis membranes.
Calcium bicarbonate, also called calcium hydrogencarbonate, has the chemical formula Ca(HCO3)2. The term does not refer to a known solid compound; it exists only in aqueous solution containing calcium (Ca2+), bicarbonate (HCO−
3), and carbonate (CO2−
3) ions, together with dissolved carbon dioxide (CO2). The relative concentrations of these carbon-containing species depend on the pH; bicarbonate predominates within the range 6.36–10.25 in fresh water.

Alkali, or Alkaline, soils are clay soils with high pH, a poor soil structure and a low infiltration capacity. Often they have a hard calcareous layer at 0.5 to 1 metre depth. Alkali soils owe their unfavorable physico-chemical properties mainly to the dominating presence of sodium carbonate, which causes the soil to swell and difficult to clarify/settle. They derive their name from the alkali metal group of elements, to which sodium belongs, and which can induce basicity. Sometimes these soils are also referred to as alkaline sodic soils. Alkaline soils are basic, but not all basic soils are alkaline.

Chloride shift (also known as the Hamburger phenomenon or lineas phenomenon, named after Hartog Jakob Hamburger) is a process which occurs in a cardiovascular system and refers to the exchange of bicarbonate (HCO3−) and chloride (Cl−) across the membrane of red blood cells (RBCs).

The residual sodium carbonate (RSC) index of irrigation water or soil water is used to indicate the alkalinity hazard for soil. The RSC index is used to find the suitability of the water for irrigation in clay soils which have a high cation exchange capacity. When dissolved sodium in comparison with dissolved calcium and magnesium is high in water, clay soil swells or undergoes dispersion which drastically reduces its infiltration capacity.

The carbonic anhydrases form a family of enzymes that catalyze the interconversion between carbon dioxide and water and the dissociated ions of carbonic acid. The active site of most carbonic anhydrases contains a zinc ion. They are therefore classified as metalloenzymes. The enzyme maintains acid-base balance and helps transport carbon dioxide.

The dry ice color show is a demonstration of the chemical formation of carbonic acid by the dissolution of dry ice in water. The dry ice color show is usually performed in classrooms to demonstrate the properties of acids and bases, their effect on pH indicators, and the sublimation of dry ice. Setup is simple and generally involves only minor hazards, the main one being the low temperature of dry ice, which can cause frostbite upon skin contact. The carbonic acid formed in the demonstration is a weak acid and is not hazardous, being present in numerous consumer products including tonic water, soda, and beer.

Total inorganic carbon is the sum of the inorganic carbon species.

Transition metal carbonate and bicarbonate complexes are coordination compounds containing carbonate (CO32-) and bicarbonate (HCO3-) as ligands. The inventory of complexes is large, enhanced by the fact that the carbonate ligand can bind metal ions in a variety of bonding modes. They illustrate the fate of low valent complexes when exposed to air.