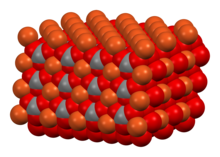
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently bound group –OH of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry.
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions.

Basic copper carbonate is a chemical compound, more properly called copper(II) carbonate hydroxide. It is an ionic compound consisting of the ions copper(II) Cu2+
, carbonate CO2−
3, and hydroxide OH−
.

In organic chemistry, Fehling's solution is a chemical reagent used to differentiate between water-soluble carbohydrate and ketone functional groups, and as a test for reducing sugars and non-reducing sugars, supplementary to the Tollens' reagent test. The test was developed by German chemist Hermann von Fehling in 1849.

Azurite is a soft, deep-blue copper mineral produced by weathering of copper ore deposits. During the early 19th century, it was also known as chessylite, after the type locality at Chessy-les-Mines near Lyon, France. The mineral, a basic carbonate with the chemical formula Cu3(CO3)2(OH)2, has been known since ancient times, and was mentioned in Pliny the Elder's Natural History under the Greek name kuanos (κυανός: "deep blue," root of English cyan) and the Latin name caeruleum. Copper (Cu2+) gives it its blue color.

Copper(II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu2O or copper(I) oxide (cuprous oxide). As a mineral, it is known as tenorite. It is a product of copper mining and the precursor to many other copper-containing products and chemical compounds.
Classical qualitative inorganic analysis is a method of analytical chemistry which seeks to find the elemental composition of inorganic compounds. It is mainly focused on detecting ions in an aqueous solution, therefore materials in other forms may need to be brought to this state before using standard methods. The solution is then treated with various reagents to test for reactions characteristic of certain ions, which may cause color change, precipitation and other visible changes.
Copper carbonate may refer to :

Copper(II) chloride, also known as cupric chloride, is an inorganic compound with the chemical formula CuCl2. The monoclinic yellowish-brown anhydrous form slowly absorbs moisture to form the orthorhombic blue-green dihydrate CuCl2·2H2O, with two water molecules of hydration. It is industrially produced for use as a co-catalyst in the Wacker process.

Copper(II) hydroxide is the hydroxide of copper with the chemical formula of Cu(OH)2. It is a pale greenish blue or bluish green solid. Some forms of copper(II) hydroxide are sold as "stabilized" copper(II) hydroxide, although they likely consist of a mixture of copper(II) carbonate and hydroxide. Cupric hydroxide is a strong base, although its low solubility in water makes this hard to observe directly.
In chemical nomenclature, the IUPAC nomenclature of inorganic chemistry is a systematic method of naming inorganic chemical compounds, as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in Nomenclature of Inorganic Chemistry. Ideally, every inorganic compound should have a name from which an unambiguous formula can be determined. There is also an IUPAC nomenclature of organic chemistry.
A solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and anions dissolve in or precipitate from solution.

Iron(II) carbonate, or ferrous carbonate, is a chemical compound with formula FeCO
3, that occurs naturally as the mineral siderite. At ordinary ambient temperatures, it is a green-brown ionic solid consisting of iron(II) cations Fe2+
and carbonate anions CO2−
3.

Copper(II) phosphate are inorganic compounds with the formula Cu3(PO4)2. They can be regarded as the cupric salts of phosphoric acid. Anhydrous copper(II) phosphate and a trihydrate are blue solids.

Acetylenedicarboxylic acid or butynedioic acid is an organic compound with the formula H2C4O4 or HO−C(=O)−C≡C−C(=O)−OH. It is a crystalline solid that is soluble in diethyl ether.
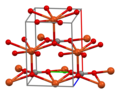
Dicopper chloride trihydroxide is the chemical compound with the formula Cu2(OH)3Cl. It is often referred to as tribasic copper chloride (TBCC), copper trihydroxyl chloride or copper hydroxychloride. It is a greenish crystalline solid encountered in mineral deposits, metal corrosion products, industrial products, art and archeological objects, and some living systems. It was originally manufactured on an industrial scale as a precipitated material used as either a chemical intermediate or a fungicide. Since 1994, a purified, crystallized product has been produced at the scale of thousands of tons per year, and used extensively as a nutritional supplement for animals.

Compounds of lead exist with lead in two main oxidation states: +2 and +4. The former is more common. Inorganic lead(IV) compounds are typically strong oxidants or exist only in highly acidic solutions.
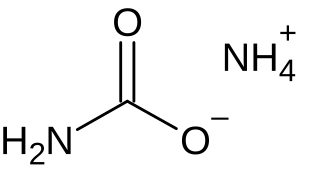
Ammonium carbamate is a chemical compound with the formula [NH4][H2NCO2] consisting of ammonium cation NH+4 and carbamate anion NH2COO−. It is a white solid that is extremely soluble in water, less so in alcohol. Ammonium carbamate can be formed by the reaction of ammonia NH3 with carbon dioxide CO2, and will slowly decompose to those gases at ordinary temperatures and pressures. It is an intermediate in the industrial synthesis of urea (NH2)2CO, an important fertilizer.

Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. Copper compounds, whether organic complexes or organometallics, promote or catalyse numerous chemical and biological processes.