
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently bound group –OH of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry.

Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations Na+ and hydroxide anions OH−.

In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rouelle in the mid-18th century.

Potassium hydroxide is an inorganic compound with the formula KOH, and is commonly called caustic potash.

Rubidium oxide is the chemical compound with the formula Rb2O. Rubidium oxide is highly reactive towards water, and therefore it would not be expected to occur naturally. The rubidium content in minerals is often calculated and quoted in terms of Rb2O. In reality, the rubidium is typically present as a component of (actually, an impurity in) silicate or aluminosilicate. A major source of rubidium is lepidolite, KLi2Al(Al,Si)3O10(F,OH)2, wherein Rb sometimes replaces K.

Copper(II) hydroxide is the hydroxide of copper with the chemical formula of Cu(OH)2. It is a pale greenish blue or bluish green solid. Some forms of copper(II) hydroxide are sold as "stabilized" copper(II) hydroxide, although they likely consist of a mixture of copper(II) carbonate and hydroxide. Cupric hydroxide is a strong base, although its low solubility in water makes this hard to observe directly.
Basic oxides are oxides that show basic properties in opposition to acidic oxides and that either

Yttrium(III) fluoride is an inorganic chemical compound with the chemical formula YF3. It is not known naturally in 'pure' form. The fluoride minerals containing essential yttrium include tveitite-(Y) (Y,Na)6Ca6Ca6F42 and gagarinite-(Y) NaCaY(F,Cl)6. Sometimes mineral fluorite contains admixtures of yttrium.

Cobalt(II) hydroxide or cobaltous hydroxide is the inorganic compound with the formula Co(OH)
2, consisting of divalent cobalt cations Co2+
and hydroxide anions HO−
. The pure compound, often called the "beta form" is a pink solid insoluble in water.

Sodium selenide is an inorganic compound of sodium and selenium with the chemical formula Na2Se.
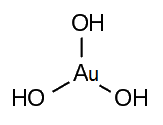
Gold(III) hydroxide, gold trihydroxide, or gold hydroxide is an inorganic compound, a hydroxide of gold, with formula Au(OH)3. It is also called auric acid with formula H3AuO3. It is easily dehydrated above 140 °C to gold(III) oxide. Salts of auric acid are termed aurates.

Sodium bismuthate is an inorganic compound, and a strong oxidiser with chemical formula NaBiO3. It is somewhat hygroscopic, but not soluble in cold water, which can be convenient since the reagent can be easily removed after the reaction. It is one of the few water insoluble sodium salts. Commercial samples may be a mixture of bismuth(V) oxide, sodium carbonate and sodium peroxide.

Compounds of lead exist with lead in two main oxidation states: +2 and +4. The former is more common. Inorganic lead(IV) compounds are typically strong oxidants or exist only in highly acidic solutions.
Yttrium(III) sulfate is an inorganic compound with the formula Y2(SO4)3. The most common form is the anhydrate and octahydrate.
An yttrium compound is a chemical compound containing yttrium. Among these compounds, yttrium generally has a +3 valence. The solubility properties of yttrium compounds are similar to those of the lanthanides. For example oxalates and carbonates are hardly soluble in water, but soluble in excess oxalate or carbonate solutions as complexes are formed. Sulfates and double sulfates are generally soluble. They resemble the "yttrium group" of heavy lanthanide elements.

Lutetium(III) hydroxide is an inorganic compound with the chemical formula Lu(OH)3.
Scandium(III) hydroxide is an inorganic compound with the chemical formula Sc(OH)3, the trivalent hydroxide of scandium. It is an amphoteric compound. It is slightly soluble in water, and its saturated solution (pH = 7.85) contains Sc(OH)3 and a small amount of Sc(OH)2+. The solubility of scandium(III) hydroxide in water is 0.0279 mol/L. It will convert to ScO(OH) after aging, greatly reducing the solubility (0.0008 mol/L). Scandium(III) hydroxide can be produced by reacting scandium salts and alkali hydroxides. In the reaction, different starting ingredients can generate different intermediates such as Sc(OH)1.75Cl1.25, Sc(OH)2NO3 and Sc(OH)2.32(SO4)0.34.
Yttrium iodide is a binary inorganic compound, a salt of yttrium and hydroiodic acid with the formula YI
3. The compound forms colorless crystals, soluble in water.
Gallium compounds compounds containing the element gallium. These compounds are found primarily in the +3 oxidation state. The +1 oxidation state is also found in some compounds, although it is less common than it is for gallium's heavier congeners indium and thallium. For example, the very stable GaCl2 contains both gallium(I) and gallium(III) and can be formulated as GaIGaIIICl4; in contrast, the monochloride is unstable above 0 °C, disproportionating into elemental gallium and gallium(III) chloride. Compounds containing Ga–Ga bonds are true gallium(II) compounds, such as GaS (which can be formulated as Ga24+(S2−)2) and the dioxan complex Ga2Cl4(C4H8O2)2.
Neptunium compounds are compounds containg the element neptunium (Np). Neptunium has five ionic oxidation states ranging from +3 to +7 when forming chemical compounds, which can be simultaneously observed in solutions. It is the heaviest actinide that can lose all its valence electrons in a stable compound. The most stable state in solution is +5, but the valence +4 is preferred in solid neptunium compounds. Neptunium metal is very reactive. Ions of neptunium are prone to hydrolysis and formation of coordination compounds.












