
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently bound group –OH of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry.

Indium is a chemical element; it has symbol In and atomic number 49. It is a silvery-white post-transition metal and one of the softest elements. Chemically, indium is similar to gallium and thallium, and its properties are largely intermediate between the two. It was discovered in 1863 by Ferdinand Reich and Hieronymous Theodor Richter by spectroscopic methods and named for the indigo blue line in its spectrum.

The boron group are the chemical elements in group 13 of the periodic table, consisting of boron (B), aluminium (Al), gallium (Ga), indium (In), thallium (Tl) and nihonium (Nh). This group lies in the p-block of the periodic table. The elements in the boron group are characterized by having three valence electrons. These elements have also been referred to as the triels.
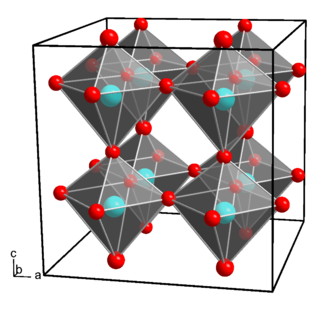
Gallium hydroxide is an inorganic compound with the chemical formula Ga(OH)3. It is formed as a gel following the addition of ammonia to Ga3+ salts. It is also found in nature as the rare mineral söhngeite which is reported to contain octahedrally coordinated gallium atoms. Gallium hydroxide is amphoteric. In strongly acidic conditions, the gallium ion, Ga3+ is formed. In strongly basic conditions, [Ga(OH)4]− (tetrahydroxogallate(III)) is formed. Salts of [Ga(OH)4]− are sometimes called gallates.

Thallium(I) oxide is the inorganic compound of thallium and oxygen with the formula Tl2O in which thallium is in its +1 oxidation state. It is black and produces a basic yellow solution of thallium(I) hydroxide (TlOH) when dissolved in water. It is formed by heating solid TlOH or Tl2CO3 in the absence of air. Thallium oxide is used to make special high refractive index glass. Thallium oxide is a component of several high temperature superconductors. Thallium(I) oxide reacts with acids to make thallium(I) salts.

Indium(III) chloride is the chemical compound with the formula InCl3 which forms a tetrahydrate. This salt is a white, flaky solid with applications in organic synthesis as a Lewis acid. It is also the most available soluble derivative of indium. This is one of three known indium chlorides.

Gallium(III) bromide (GaBr3) is a chemical compound, and one of four gallium trihalides.

Gallium(III) fluoride (GaF3) is a chemical compound. It is a white solid that melts under pressure above 1000 °C but sublimes around 950 °C. It has the FeF3 structure where the gallium atoms are 6-coordinate. GaF3 can be prepared by reacting F2 or HF with Ga2O3 or by thermal decomposition of (NH4)3GaF6. GaF3 is virtually insoluble in water. Solutions of GaF3 in HF can be evaporated to form the trihydrate, GaF3·3H2O, which on heating gives a hydrated form of GaF2(OH). Gallium(III) fluoride reacts with mineral acids to form hydrofluoric acid.

Gallium(III) oxide is an inorganic compound and ultra-wide-bandgap semiconductor with the formula Ga2O3. It is actively studied for applications in power electronics, phosphors, and gas sensing. The compound has several polymorphs, of which the monoclinic β-phase is the most stable. The β-phase’s bandgap of 4.7–4.9 eV and large-area, native substrates make it a promising competitor to GaN and SiC-based power electronics applications and solar-blind UV photodetectors. The orthorhombic ĸ-Ga2O3 is the second most stable polymorph. The ĸ-phase has shown instability of subsurface doping density under thermal exposure. Ga2O3 exhibits reduced thermal conductivity and electron mobility by an order of magnitude compared to GaN and SiC, but is predicted to be significantly more cost-effective due to being the only wide-bandgap material capable of being grown from melt. β-Ga2O3 is thought to be radiation-hard, which makes it promising for military and space applications.
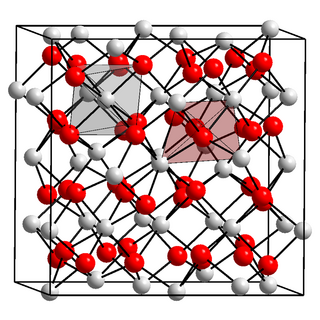
Indium(III) oxide (In2O3) is a chemical compound, an amphoteric oxide of indium.
Indium(III) sulfate (In2(SO4)3) is a sulfate salt of the metal indium. It is a sesquisulfate, meaning that the sulfate group occurs 11/2 times as much as the metal. It may be formed by the reaction of indium, its oxide, or its carbonate with sulfuric acid. An excess of strong acid is required, otherwise insoluble basic salts are formed. As a solid indium sulfate can be anhydrous, or take the form of a pentahydrate with five water molecules or a nonahydrate with nine molecules of water. Indium sulfate is used in the production of indium or indium containing substances. Indium sulfate also can be found in basic salts, acidic salts or double salts including indium alum.

Gallium(III) chloride is an inorganic chemical compound with the formula GaCl3 which forms a monohydrate, GaCl3·H2O. Solid gallium(III) chloride is a deliquescent white solid and exists as a dimer with the formula Ga2Cl6. It is colourless and soluble in virtually all solvents, even alkanes, which is truly unusual for a metal halide. It is the main precursor to most derivatives of gallium and a reagent in organic synthesis.
Aluminium carbonate (Al2(CO3)3), is a carbonate of aluminium. It is not well characterized; one authority says that simple carbonates of aluminium are not known. However related compounds are known, such as the basic sodium aluminium carbonate mineral dawsonite (NaAlCO3(OH)2) and hydrated basic aluminium carbonate minerals scarbroite (Al5(CO3)(OH)13•5(H2O)) and hydroscarbroite (Al14(CO3)3(OH)36•nH2O).
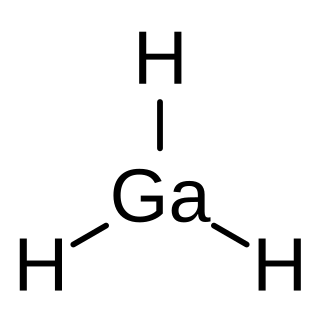
Gallane, also systematically named trihydridogallium, is an inorganic compound of gallium with the chemical formula GaH
3. It is a photosensitive, colourless gas that cannot be concentrated in pure form. Gallane is both the simplest member of the gallanes, and the prototype of the monogallanes. It has no economic uses, and is only intentionally produced for academic reasons.

Gallium(III) sulfide, Ga2S3, is a compound of sulfur and gallium, that is a semiconductor that has applications in electronics and photonics.

The metallic elements in the periodic table located between the transition metals to their left and the chemically weak nonmetallic metalloids to their right have received many names in the literature, such as post-transition metals, poor metals, other metals, p-block metals, basic metals, and chemically weak metals. The most common name, post-transition metals, is generally used in this article.
Aluminium triacetate, formally named aluminium acetate, is a chemical compound with composition Al(CH
3CO
2)
3. Under standard conditions it appears as a white, water-soluble solid that decomposes on heating at around 200 °C. The triacetate hydrolyses to a mixture of basic hydroxide / acetate salts, and multiple species co-exist in chemical equilibrium, particularly in aqueous solutions of the acetate ion; the name aluminium acetate is commonly used for this mixed system.
Gallium(III) sulfate refers to the chemical compound, a salt, with the formula Ga2(SO4)3, or its hydrates Ga2(SO4)3·xH2O. Gallium metal dissolves in sulfuric acid to form solutions containing [Ga(OH2)6]3+ and SO42− ions. The octadecahydrate Ga2(SO4)3·18H2O crystallises from these solutions at room temperature. This hydrate loses water in stages when heated, forming the anhydrate Ga2(SO4)3 above 150 °C and completely above 310 °C. Anhydrous Ga2(SO4)3 is isostructural with iron(III) sulfate, crystallizing in the rhombohedral space group R3.
Gallium compounds are compounds containing the element gallium. These compounds are found primarily in the +3 oxidation state. The +1 oxidation state is also found in some compounds, although it is less common than it is for gallium's heavier congeners indium and thallium. For example, the very stable GaCl2 contains both gallium(I) and gallium(III) and can be formulated as GaIGaIIICl4; in contrast, the monochloride is unstable above 0 °C, disproportionating into elemental gallium and gallium(III) chloride. Compounds containing Ga–Ga bonds are true gallium(II) compounds, such as GaS (which can be formulated as Ga24+(S2−)2) and the dioxan complex Ga2Cl4(C4H8O2)2. There are also compounds of gallium with negative oxidation states, ranging from -5 to -1, most of these compounds being magnesium gallides (MgxGay).
Ammonium hexafluoroindate is an inorganic chemical compound with the chemical formula (NH4)3InF6.














