
Iron(III) chloride is the inorganic compound with the formula FeCl3. Also called ferric chloride, it is a common compound of iron in the +3 oxidation state. The anhydrous compound is a crystalline solid with a melting point of 307.6 °C. The colour depends on the viewing angle: by reflected light the crystals appear dark green, but by transmitted light they appear purple-red.
Neodymium(III) chloride or neodymium trichloride is a chemical compound of neodymium and chlorine with the formula NdCl3. This anhydrous compound is a mauve-colored solid that rapidly absorbs water on exposure to air to form a purple-colored hexahydrate, NdCl3·6H2O. Neodymium(III) chloride is produced from minerals monazite and bastnäsite using a complex multistage extraction process. The chloride has several important applications as an intermediate chemical for production of neodymium metal and neodymium-based lasers and optical fibers. Other applications include a catalyst in organic synthesis and in decomposition of waste water contamination, corrosion protection of aluminium and its alloys, and fluorescent labeling of organic molecules (DNA).

Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula AlCl3. It forms hexahydrate with the formula [Al(H2O)6]Cl3, containing six water molecules of hydration. Both are colourless crystals, but samples are often contaminated with iron(III) chloride, giving a yellow color.

Rhodium(III) chloride refers to inorganic compounds with the formula RhCl3(H2O)n, where n varies from 0 to 3. These are diamagnetic solids featuring octahedral Rh(III) centres. Depending on the value of n, the material is either a dense brown solid or a soluble reddish salt. The soluble trihydrated (n = 3) salt is widely used to prepare compounds used in homogeneous catalysis, notably for the industrial production of acetic acid and hydroformylation.

Gold(III) chloride, traditionally called auric chloride, is a compound of gold and chlorine with the molecular formula Au2Cl6. The "III" in the name indicates that the gold has an oxidation state of +3, typical for many gold compounds. Gold(III) chloride is hygroscopic and decomposes in visible light. This compound is a dimer of AuCl3. This compound has few uses, although it catalyzes various organic reactions.
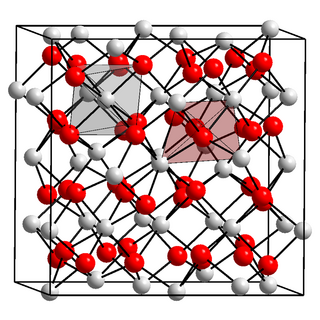
Scandium(III) oxide or scandia is a inorganic compound with formula Sc2O3. It is one of several oxides of rare earth elements with a high melting point. It is used in the preparation of other scandium compounds as well as in high-temperature systems (for its resistance to heat and thermal shock), electronic ceramics, and glass composition (as a helper material).
Iron(III) oxide-hydroxide or ferric oxyhydroxide is the chemical compound of iron, oxygen, and hydrogen with formula FeO(OH).
Basic oxides are oxides that show basic properties in opposition to acidic oxides and that either
Calcium chlorate is the calcium salt of chloric acid, with the chemical formula Ca(ClO3)2. Like other chlorates, it is a strong oxidizer.
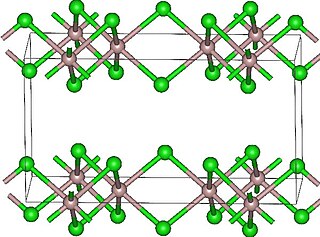
Lutetium(III) chloride or lutetium trichloride is the chemical compound composed of lutetium and chlorine with the formula LuCl3. It forms hygroscopic white monoclinic crystals and also a hydroscopic hexahydrate LuCl3·6H2O. Anhydrous lutetium(III) chloride has the YCl3 (AlCl3) layer structure with octahedral lutetium ions.

Cadmium hydroxide is an inorganic compound with the formula Cd(OH)2. It is a white crystalline ionic compound that is a key component of nickel–cadmium battery.

Sodium hexachloroplatinate(IV), the sodium salt of chloroplatinic acid, is an inorganic compound with the formula Na2[PtCl6], consisting of the sodium cation and the hexachloroplatinate anion. As explained by Cox and Peters, anhydrous sodium hexachloroplatinate, which is yellow, tends to form the orange hexahydrate upon storage in humid air. The latter can be dehydrated upon heating at 110 °C.

Berkelium forms a number of chemical compounds, where it normally exists in an oxidation state of +3 or +4, and behaves similarly to its lanthanide analogue, terbium. Like all actinides, berkelium easily dissolves in various aqueous inorganic acids, liberating gaseous hydrogen and converting into the trivalent oxidation state. This trivalent state is the most stable, especially in aqueous solutions, but tetravalent berkelium compounds are also known. The existence of divalent berkelium salts is uncertain and has only been reported in mixed lanthanum chloride-strontium chloride melts. Aqueous solutions of Bk3+ ions are green in most acids. The color of the Bk4+ ions is yellow in hydrochloric acid and orange-yellow in sulfuric acid. Berkelium does not react rapidly with oxygen at room temperature, possibly due to the formation of a protective oxide surface layer; however, it reacts with molten metals, hydrogen, halogens, chalcogens and pnictogens to form various binary compounds. Berkelium can also form several organometallic compounds.

Metal halides are compounds between metals and halogens. Some, such as sodium chloride are ionic, while others are covalently bonded. A few metal halides are discrete molecules, such as uranium hexafluoride, but most adopt polymeric structures, such as palladium chloride.

Lutetium(III) fluoride is an inorganic compound with a chemical formula LuF3.

Berkelium(III) chloride also known as berkelium trichloride, is a chemical compound with the formula BkCl3. It is a water-soluble green solid with a melting point of 603 °C. This compound forms the hexahydrate, BkCl3·6H2O.

Terbium compounds are compounds formed by the lanthanide metal terbium (Tb). Terbium generally exhibits the +3 oxidation state in these compounds, such as in TbCl3, Tb(NO3)3 and Tb(CH3COO)3. Compounds with terbium in the +4 oxidation state are also known, such as TbO2 and BaTbF6. Terbium can also form compounds in the 0, +1 and +2 oxidation states.
Cobalt compounds are chemical compounds formed by cobalt with other elements. In the compound, the most stable oxidation state of cobalt is the +2 oxidation state, and in the presence of specific ligands, there are also stable compounds with +3 valence. In addition, there are cobalt compounds in high oxidation states +4, +5 and low oxidation states -1, 0, +1.
Gallium compounds compounds containing the element gallium. These compounds are found primarily in the +3 oxidation state. The +1 oxidation state is also found in some compounds, although it is less common than it is for gallium's heavier congeners indium and thallium. For example, the very stable GaCl2 contains both gallium(I) and gallium(III) and can be formulated as GaIGaIIICl4; in contrast, the monochloride is unstable above 0 °C, disproportionating into elemental gallium and gallium(III) chloride. Compounds containing Ga–Ga bonds are true gallium(II) compounds, such as GaS (which can be formulated as Ga24+(S2−)2) and the dioxan complex Ga2Cl4(C4H8O2)2.
Neptunium compounds are compounds containg the element neptunium (Np). Neptunium has five ionic oxidation states ranging from +3 to +7 when forming chemical compounds, which can be simultaneously observed in solutions. It is the heaviest actinide that can lose all its valence electrons in a stable compound. The most stable state in solution is +5, but the valence +4 is preferred in solid neptunium compounds. Neptunium metal is very reactive. Ions of neptunium are prone to hydrolysis and formation of coordination compounds.














