
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colorless, acidic and highly corrosive. A common concentration is 49% (48-52%) but there are also stronger solutions and pure HF has a boiling point near room temperature. It is used to make most fluorine-containing compounds; examples include the commonly used pharmaceutical antidepressant medication fluoxetine (Prozac) and the material PTFE (Teflon). Elemental fluorine is produced from it. It is commonly used to etch glass and silicon wafers.
In chemistry, an interhalogen compound is a molecule which contains two or more different halogen atoms and no atoms of elements from any other group.
Chlorine trifluoride is an interhalogen compound with the formula ClF3. It is a colorless, poisonous, corrosive, and extremely reactive gas that condenses to a pale-greenish yellow liquid, the form in which it is most often sold. It is famous for its extreme oxidation properties. The compound is primarily of interest in plasmaless cleaning and etching operations in the semiconductor industry, in nuclear reactor fuel processing, historically as a component in rocket fuels, and various other industrial operations owing to its corrosive nature.

Manganese(III) fluoride (also known as Manganese trifluoride) is the inorganic compound with the formula MnF3. This red/purplish solid is useful for converting hydrocarbons into fluorocarbons, i.e., it is a fluorination agent. It forms a hydrate and many derivatives.

Chlorine pentafluoride is an interhalogen compound with formula ClF5. This colourless gas is a strong oxidant that was once a candidate oxidizer for rockets. The molecule adopts a square pyramidal structure with C4v symmetry, as confirmed by its high-resolution 19F NMR spectrum. It was first synthesized in 1963.
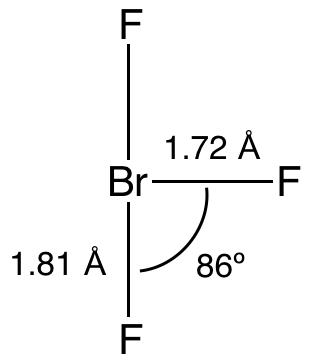
Bromine trifluoride is an interhalogen compound with the formula BrF3. At room temperature, it is a straw-coloured liquid with a pungent odor which decomposes violently on contact with water and organic compounds. It is a powerful fluorinating agent and an ionizing inorganic solvent. It is used to produce uranium hexafluoride (UF6) in the processing and reprocessing of nuclear fuel.

Chromium(III) fluoride is an inorganic compound with the chemical formula CrF3. It forms several hydrates. The compound CrF3 is a green crystalline solid that is insoluble in common solvents, but the hydrates [Cr(H2O)6]F3 (violet) and [Cr(H2O)6]F3·3H2O (green) are soluble in water. The anhydrous form sublimes at 1100–1200 °C.

Cobalt(III) fluoride is the inorganic compound with the formula CoF3. Hydrates are also known. The anhydrous compound is a hygroscopic brown solid. It is used to synthesize organofluorine compounds.

Iron(III) fluoride, also known as ferric fluoride, are inorganic compounds with the formula FeF3(H2O)x where x = 0 or 3. They are mainly of interest by researchers, unlike the related iron(III) chloride. Anhydrous iron(III) fluoride is white, whereas the hydrated forms are light pink.

Hydrogen fluoride (fluorane) is an inorganic compound with chemical formula HF. It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils at near room temperature, which is much higher of a temperature than other hydrogen halides.

Sulfur tetrafluoride is a chemical compound with the formula SF4. It is a colorless corrosive gas that releases dangerous hydrogen fluoride gas upon exposure to water or moisture. Sulfur tetrafluoride is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries.

Bismuth(III) fluoride or bismuth trifluoride is a chemical compound of bismuth and fluorine. The chemical formula is BiF3. It is a grey-white powder melting at 649 °C.

Thiophosphoryl fluoride is an inorganic molecular gas with formula PSF3 containing phosphorus, sulfur and fluorine. It spontaneously ignites in air and burns with a cool flame. The discoverers were able to have flames around their hands without discomfort, and called it "probably one of the coldest flames known". The gas was discovered in 1888.

Neptunium(VI) fluoride (NpF6) is the highest fluoride of neptunium, it is also one of seventeen known binary hexafluorides. It is a volatile orange crystalline solid. It is relatively hard to handle, being very corrosive, volatile and radioactive. Neptunium hexafluoride is stable in dry air but reacts vigorously with water.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.
Thullium(III) fluoride is an inorganic compound with the chemical formula TmF3.

Terbium(IV) fluoride is an inorganic compound with a chemical formula TbF4. It is a white solid that is a strong oxidizer. It is also a strong fluorinating agent, emitting relatively pure atomic fluorine when heated, rather than the mixture of fluoride vapors emitted from cobalt(III) fluoride or cerium(IV) fluoride.

Protactinium(V) fluoride is a fluoride of protactinium with the chemical formula PaF5.
Lutetium compounds are compounds formed by the lanthanide metal lutetium (Lu). In these compounds, lutetium generally exhibits the +3 oxidation state, such as LuCl3, Lu2O3 and Lu2(SO4)3. Aqueous solutions of most lutetium salts are colorless and form white crystalline solids upon drying, with the common exception of the iodide. The soluble salts, such as nitrate, sulfate and acetate form hydrates upon crystallization. The oxide, hydroxide, fluoride, carbonate, phosphate and oxalate are insoluble in water.
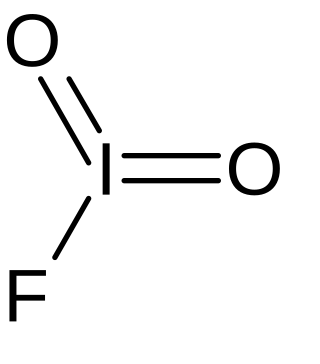
Iodyl fluoride is an inorganic compound of iodine, fluorine, and oxygen with the chemical formula IO2F. The compound was initially synthesized in 1951.














