
Samarium(III) chloride, also known as samarium trichloride, is an inorganic compound of samarium and chloride. It is a pale yellow salt that rapidly absorbs water to form a hexahydrate, SmCl3.6H2O. The compound has few practical applications but is used in laboratories for research on new compounds of samarium.

Cobalt(III) fluoride is the inorganic compound with the formula CoF3. Hydrates are also known. The anhydrous compound is a hygroscopic brown solid. It is used to synthesize organofluorine compounds.

Iron(III) fluoride, also known as ferric fluoride, are inorganic compounds with the formula FeF3(H2O)x where x = 0 or 3. They are mainly of interest by researchers, unlike the related iron(III) chloride. Anhydrous iron(III) fluoride is white, whereas the hydrated forms are light pink.

Samarium(III) oxide (Sm2O3) is a chemical compound. Samarium oxide readily forms on the surface of samarium metal under humid conditions or temperatures in excess of 150°C in dry air. Similar to rust on metallic iron, this oxide layer spalls off the surface of the metal, exposing more metal to continue the reaction. The oxide is commonly white to off yellow in color and is often encountered as a highly fine dust like powder.
Dysprosium(III) fluoride is an inorganic compound of dysprosium with a chemical formula DyF3.
Samarium(II) fluoride is one of fluorides of samarium with a chemical formula SmF2. The compound crystalizes in the fluorite structure, and is significantly nonstoichiometric. Along with europium(II) fluoride and ytterbium(II) fluoride, it is one of three known rare earth difluorides, the rest are unstable.
Terbium(III) fluoride is an inorganic compound with chemical formula TbF3. It is hard to dissolve in water. It can be produced by reacting terbium(III) carbonate and 40% hydrofluoric acid at 40°C.

Samarium(III) hydroxide is an inorganic compound with chemical formula Sm(OH)3.

Terbium(IV) fluoride is an inorganic compound with a chemical formula TbF4. It is a white solid that is a strong oxidizer. It is also a strong fluorinating agent, emitting relatively pure atomic fluorine when heated, rather than the mixture of fluoride vapors emitted from cobalt(III) fluoride or cerium(IV) fluoride. It can be produced by the reaction between very pure terbium(III) fluoride and xenon difluoride, chlorine trifluoride or fluorine gas:

Praseodymium(IV) fluoride (also praseodymium tetrafluoride) is a binary inorganic compound, a highly oxidised metal salt of praseodymium and fluoride with the chemical formula PrF4.
Samarium(III) oxalate is an inorganic compound, a salt of samarium and oxalic acid with the formula Sm2(C2O4)3. The compound does not dissolve in water, forms a crystalline hydrate with yellow crystals.
Platinum-samarium is a binary inorganic compound of platinum and samarium with the chemical formula PtSm. This intermetallic compound forms crystals.
Plutonium silicide is a binary inorganic compound of plutonium and silicon with the chemical formula PuSi. The compound forms gray crystals.

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Many europium compounds fluoresce under ultraviolet light due to the excitation of electrons to higher energy levels. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.

Terbium compounds are compounds formed by the lanthanide metal terbium (Tb). Terbium generally exhibits the +3 oxidation state in these compounds, such as in TbCl3, Tb(NO3)3 and Tb(CH3COO)3. Compounds with terbium in the +4 oxidation state are also known, such as TbO2 and BaTbF6. Terbium can also form compounds in the 0, +1 and +2 oxidation states.
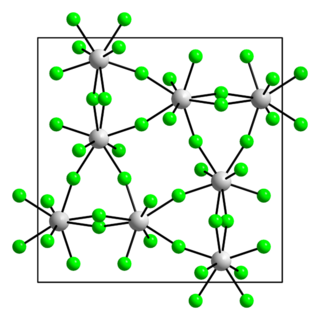
Protactinium(V) fluoride is a fluoride of protactinium with the chemical formula PaF5.
Erbium compounds are compounds containing the element erbium (Er). These compounds are usually dominated by erbium in the +3 oxidation state, although the +2, +1 and 0 oxidation states have also been reported.
Samarium(III) molybdate is an inorganic compound, with the chemical formula Sm2(MoO4)3. It is one of the compounds formed by the three elements samarium, molybdenum and oxygen.

Samarium(III) phosphate is an inorganic compound, with the chemical formula of SmPO4. It is one of the phosphates of samarium.













