   | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
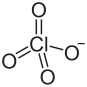
| Cl3O12Sm | |
| Molar mass | 448.70 g·mol−1 |
| Appearance | white solid [1] pale yellow crystals [2] |
| soluble in water and ethanol [2] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Samarium(III) perchlorate is an inorganic compound with the chemical formula Sm(ClO4)3.