
Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture.
Selenic acid is the inorganic compound with the formula H2SeO4. It is an oxoacid of selenium, and its structure is more accurately described as O2Se(OH)2. It is a colorless compound. Although it has few uses, one of its salts, sodium selenate is used in the production of glass and animal feeds.

Gold compounds are compounds by the element gold (Au). Although gold is the most noble of the noble metals, it still forms many diverse compounds. The oxidation state of gold in its compounds ranges from −1 to +5, but Au(I) and Au(III) dominate its chemistry. Au(I), referred to as the aurous ion, is the most common oxidation state with soft ligands such as thioethers, thiolates, and organophosphines. Au(I) compounds are typically linear. A good example is Au(CN)−2, which is the soluble form of gold encountered in mining. The binary gold halides, such as AuCl, form zigzag polymeric chains, again featuring linear coordination at Au. Most drugs based on gold are Au(I) derivatives.

Gold(V) fluoride is the inorganic compound with the formula Au2F10. This fluoride compound features gold in its highest known oxidation state. This red solid dissolves in hydrogen fluoride but these solutions decompose, liberating fluorine.

Chloro(triphenylphosphine)gold(I) or triphenylphosphinegold(I) chloride is a coordination complex with the formula (Ph3P)AuCl. This colorless solid is a common reagent for research on gold compounds.
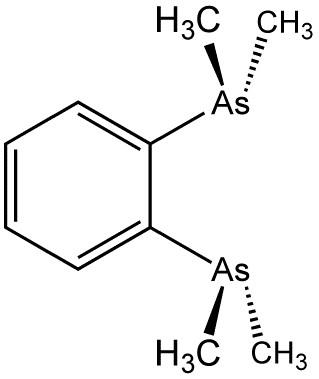
1,2-Bis(dimethylarsino)benzene (diars) is the organoarsenic compound with the formula C6H4(As(CH3)2)2. The molecule consists of two dimethylarsino groups attached to adjacent carbon centers of a benzene ring. It is a chelating ligand in coordination chemistry. This colourless oil is commonly abbreviated "diars."

Dichlorine hexoxide is the chemical compound with the molecular formula Cl
2O
6, which is correct for its gaseous state. However, in liquid or solid form, this chlorine oxide ionizes into the dark red ionic compound chloryl perchlorate [ClO
2]+
[ClO
4]−
, which may be thought of as the mixed anhydride of chloric and perchloric acids.

In chemistry, chloryl refers to a triatomic cation with chemical formula ClO+
2. This species has the same general structure as chlorite (ClO−
2) but it is electronically different, with chlorine having a +5 oxidation state (rather than the +3 of chlorite). This makes it a rare example of a positively charged oxychloride. Chloryl compounds, such as FClO
2 and [ClO2][RuF6], are all highly reactive and react violently with water and most organic compounds.

Tetrabromoauric acid is an inorganic compound with the formula H[AuBr4]. It is the bromide analog of chloroauric acid. It is generated analogously, by reacting a mixture of hydrobromic and nitric acids with elemental gold. The oxidation state of gold in H[AuBr4] and [AuBr4]− anion is +3. The salts of H[AuBr4] are tetrabromoaurates(III), containing [AuBr4]− anions, which have square planar molecular geometry.
In chemistry, molecular oxohalides (oxyhalides) are a group of chemical compounds in which both oxygen and halogen atoms are attached to another chemical element A in a single molecule. They have the general formula AOmXn, where X is a halogen. Known oxohalides have fluorine (F), chlorine (Cl), bromine (Br), and/or iodine (I) in their molecules. The element A may be a main group element, a transition element, a rare earth element or an actinide. The term oxohalide, or oxyhalide, may also refer to minerals and other crystalline substances with the same overall chemical formula, but having an ionic structure.
Perchloratoborate is an anion of the form [B(ClO4)4]−. It can form partly stable solid salts with heavy alkali metals. They are more stable than nitratoborate salts. K[B(ClO4)4] decomposes at 35 °C, Rb[B(ClO4)4] is stable to 50 °C, and Cs[B(ClO4)4] can exist up to 80 °C.

Titanium perchlorate is a molecular compound of titanium and perchlorate groups with formula Ti(ClO4)4. Anhydrous titanium perchlorate decomposes explosively at 130 °C and melts at 85 °C with a slight decomposition. It can sublime in a vacuum as low as 70 °C, and can form vapour at up to 120°. Titanium perchlorate is quite volatile. It has density 2.35. It decomposes to TiO2, ClO2 and dioxygen O2 Also TiO(ClO4)2 is formed during decomposition.
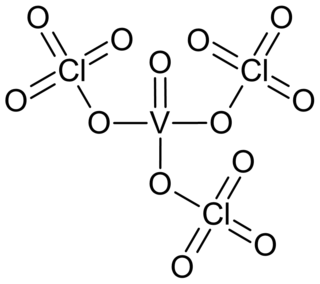
Vanadyl perchlorate or vanadyl triperchlorate is a golden yellow coloured liquid or crystalline compound of vanadium, oxygen and perchlorate group. The substance consists of molecules covalently bound and is quite volatile; it ignites organic solvents on contact and explodes at temperatures above 80 °C.

Transition metal pyridine complexes encompass many coordination complexes that contain pyridine as a ligand. Most examples are mixed-ligand complexes. Many variants of pyridine are also known to coordinate to metal ions, such as the methylpyridines, quinolines, and more complex rings.

Rhodium(III) perchlorate refers to the inorganic compound with the formula Rh(H2O)6(ClO4)3. It is a hygroscopic yellow solid. It is the perchlorate salt of the tricationic aquo complex [Rh(H2O)6]3+. The compound is prepared by treating hydrated rhodium(III) chloride and perchloric acid at elevated temperatures:
Curium compounds are compounds containing the element curium (Cm). Curium usually forms compounds in the +3 oxidation state, although compounds with curium in the +4, +5 and +6 oxidation states are also known.
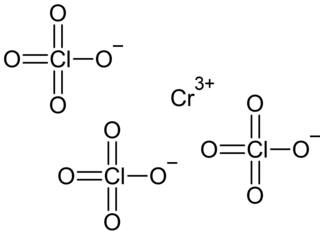
Chromium(III) perchlorate is an inorganic compound with the chemical formula Cr(ClO4)3. It's hexahydrate Cr(ClO4)3·6H2O is a cyan solid that dissolves in water.

In organic chemistry, thia-crown ethers are organosulfur compounds which are the thia analogues of crown ethers. That is, they have a sulfur atom in place of each oxygen atom around the ring. While the parent crown ethers have the formulae (CH2CH2O)n, the parent thia-crown ethers have the formulae (CH2CH2S)n, where n = 3, 4, 5, 6. They have trivial names "x-ane-Sy", where x and y are the number of atoms in the ring and the number of those atoms that are sulfur, respectively. Thia-crown ethers exhibit affinities for transition metals.

Nickel(II) perchlorate is a inorganic compound with the chemical formula of Ni(ClO4)2, and it is a strong oxidizing agent. Its colours are different depending on water. For example, the hydrate forms cyan crystals, the pentahydrate forms green crystals, but the hexahydrate (Ni(ClO4)2·6H2O) forms blue crystals.
Samarium(III) perchlorate is an inorganic compound with the chemical formula Sm(ClO4)3.














