 | |
 | |
| Names | |
|---|---|
| IUPAC name Lithium perchlorate | |
| Other names Perchloric acid, lithium salt; Lithium Cloricum | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.029.307 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| LiClO 4 | |
| Molar mass |
|
| Appearance | White crystals |
| Odor | Odorless |
| Density | 2.29 g/cm3 (20 °C (68 °F)) [1] |
| Melting point | 236 °C (457 °F; 509 K) |
| Boiling point | 430 °C (806 °F; 703 K) decomposes from 400 °C (752 °F) |
| |
| Solubility |
|
| Solubility in acetone | 137 g/100 g [2] |
| Solubility in methanol | 182 g/100 g |
| Solubility in ethanol | 152 g/100 g |
| Solubility in 1-propanol | 105 g/100 g |
| Solubility in ethyl acetate | 95.2 g/100 g [3] |
| Solubility in ethyl ether | 113.7 g/100 g [3] |
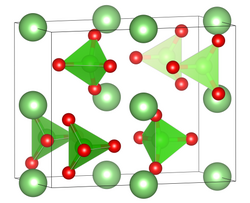
| Structure | |
| Pnma, No. 62 | |
Formula units (Z) | 4 formula per cell |
| tetrahedral at Cl | |
| Thermochemistry | |
Heat capacity (C) | 105 J/mol⋅K [2] |
Std molar entropy (S⦵298) | 125.5 J/mol⋅K [2] |
Std enthalpy of formation (ΔfH⦵298) | −380.99 kJ/mol |
Gibbs free energy (ΔfG⦵) | −254 kJ/mol [2] |
| Hazards [1] | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | Oxidizer, irritant |
| GHS labelling: | |
   | |
| Danger | |
| H272, H315, H319, H335 | |
| P210, P220, P221, P260, P264, P270, P271, P280, P301+P312+P330, P301+P330+P331, P303+P361+P353, P304+P340+P310, P305+P351+P338+P310, P363, P370+P378, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) | 1150+850 −850 mg/kg (Oral - Rat - Female) |
| Safety data sheet (SDS) | MSDS |
| Related compounds | |
Other anions | |
Other cations | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Lithium perchlorate is the inorganic compound with the formula LiClO4. This white or colourless crystalline salt is noteworthy for its high solubility in many solvents. It exists both in anhydrous form and as a trihydrate.
