Related Research Articles

Ammonium perchlorate ("AP") is an inorganic compound with the formula NH4ClO4. It is a colorless or white solid that is soluble in water. It is a powerful oxidizer. Combined with a fuel, it can be used as a rocket propellant called ammonium perchlorate composite propellant. Its instability has involved it in a number of accidents, such as the PEPCON disaster.

Perchloric acid is a mineral acid with the formula HClO4. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid, nitric acid and hydrochloric acid. It is a powerful oxidizer when hot, but aqueous solutions up to approximately 70% by weight at room temperature are generally safe, only showing strong acid features and no oxidizing properties. Perchloric acid is useful for preparing perchlorate salts, especially ammonium perchlorate, an important rocket fuel component. Perchloric acid is dangerously corrosive and readily forms potentially explosive mixtures.
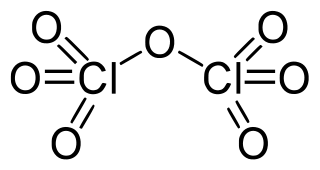
Dichlorine heptoxide is the chemical compound with the formula Cl2O7. This chlorine oxide is the anhydride of perchloric acid. It is produced by the careful distillation of perchloric acid in the presence of the dehydrating agent phosphorus pentoxide:

Sodium perchlorate is an inorganic compound with the chemical formula NaClO4. It consists of sodium cations Na+ and perchlorate anions ClO−4. It is a white crystalline, hygroscopic solid that is highly soluble in water and ethanol. It is usually encountered as sodium perchlorate monohydrate NaClO4·H2O. The compound is noteworthy as the most water-soluble of the common perchlorate salts.

Silver perchlorate is the chemical compound with the formula AgClO4. This white solid forms a monohydrate and is mildly deliquescent. It is a useful source of the Ag+ ion, although the presence of perchlorate presents risks. It is used as a catalyst in organic chemistry.

Fluorine perchlorate, also called perchloryl hypofluorite is the rarely encountered chemical compound of fluorine, chlorine, and oxygen with the chemical formula ClO
4F or FOClO
3. It is an extremely unstable gas that explodes spontaneously and has a penetrating odor.

Dichlorine hexoxide is the chemical compound with the molecular formula Cl
2O
6, which is correct for its gaseous state. However, in liquid or solid form, this chlorine oxide ionizes into the dark red ionic compound chloryl perchlorate [ClO
2]+
[ClO
4]−
, which may be thought of as the mixed anhydride of chloric and perchloric acids. This compound is a notable perchlorating agent.

Chlorine perchlorate is a chemical compound with the formula Cl2O4. This chlorine oxide is an asymmetric oxide, with one chlorine atom in +1 oxidation state and the other +7, with proper formula ClOClO3. It is produced by the photodimerization of chlorine dioxide (ClO2) at room temperature by 436 nm ultraviolet light:
Barium perchlorate is a powerful oxidizing agent, with the formula Ba(ClO4)2. It is used in the pyrotechnic industry.
Perchloratoborate is an anion of the form [B(ClO4)4]−. It can form partly stable solid salts with heavy alkali metals. They are more stable than nitratoborate salts. K[B(ClO4)4] decomposes at 35 °C, Rb[B(ClO4)4] is stable to 50 °C, and Cs[B(ClO4)4] can exist up to 80 °C.
Titanium perchlorate is a molecular compound of titanium and perchlorate groups with formula Ti(ClO4)4. Anhydrous titanium perchlorate decomposes explosively at 130 °C and melts at 85 °C with a slight decomposition. It sublimes in a vacuum as low as 70 °C. Being a molecular with four perchlorate ligands, it is an unusual example of a transition metal perchlorate complex.

Zirconium perchlorate is an inorganic compound with the formula Zr(ClO4)4. It is a hygroscopic colorless solid that sublimes in a vacuum at 70 °C. These properties show that the compound is covalently bonded molecule, rather than a salt. It is an example of a transition metal perchlorate complex.
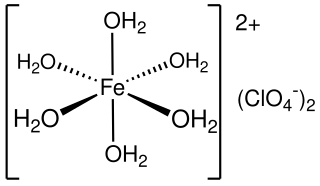
Iron(II) perchlorate is the inorganic compound with the formula Fe(ClO4)2·6H2O. A green, water-soluble solid, it is produced by the reaction of iron metal with dilute perchloric acid followed by evaporation of the solution:

Rhodium(III) perchlorate refers to the inorganic compound with the formula Rh(H2O)6(ClO4)3. It is a hygroscopic yellow solid. It is the perchlorate salt of the tricationic aquo complex [Rh(H2O)6]3+. The compound is prepared by treating hydrated rhodium(III) chloride and perchloric acid at elevated temperatures:
Hydronium perchlorate is an inorganic chemical compound with the chemical formula [H3O]ClO4. It is an unusual salt due to it being a solid and stable hydronium salt. It consists of hydronium cations [H3O]+ and perchlorate anions ClO−4.

Nickel(II) perchlorate is a collection of inorganic compounds with the chemical formula of Ni(ClO4)2(H2O)x. Its colors of these solids vary with the degree of hydration. For example, the hydrate forms cyan crystals, the pentahydrate forms green crystals, but the hexahydrate (Ni(ClO4)2·6H2O) forms blue crystals. Nickel(II) perchlorate hexahydrate is highly soluble in water and soluble in some polar organic solvents.

Lead(II) perchlorate is a chemical compound with the formula Pb(ClO4)2·xH2O, where is x is 0,1, or 3. It is an extremely hygroscopic white solid that is very soluble in water.

Nitrosyl perchlorate is the inorganic compound with the formula NO(ClO4). A hygroscopic white solid, it is the salt of the nitrosonium cation with the perchlorate anion. It is an oxidant and strong electrophile, but has fallen out of use with the availability of the closely related salt nitrosonium tetrafluoroborate NO(BF4).

Cobalt(II) perchlorate is an inorganic chemical compound with the formula Co(ClO4)2·nH2O (n = 0,6). The pink anhydrous and red hexahydrate forms are both hygroscopic solids.

Transition metal perchlorate complexes are coordination complexes with one or more perchlorate ligands. Perchlorate can bind to metals through one, two, three, or all four oxygen atoms. Usually however, perchlorate is a counterion, not a ligand.
References
- 1 2 3 4 5 6 7 8 Babaeva, V. P.; Rosolovskij, V. Ya. (1984). "Anhydrous niobium(V) perchlorate and perchloratoniobates". Russian Journal of Inorganic Chemistry. 29 (11): 1566–1568. Retrieved 5 December 2023.
- ↑ Berg, Rolf W. (1992). "Progress in Niobium and Tantalum coordination chemistry". Coordination Chemistry Reviews. 113: 1–130. doi:10.1016/0010-8545(92)80074-2.