
An explosive is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An explosive charge is a measured quantity of explosive material, which may either be composed solely of one ingredient or be a mixture containing at least two substances.

A solid-propellant rocket or solid rocket is a rocket with a rocket engine that uses solid propellants (fuel/oxidizer). The earliest rockets were solid-fuel rockets powered by gunpowder. The inception of gunpowder rockets in warfare can be credited to the ancient Chinese, and in the 13th century, the Mongols played a pivotal role in facilitating their westward adoption.

Ammonium perchlorate ("AP") is an inorganic compound with the formula NH4ClO4. It is a colorless or white solid that is soluble in water. It is a powerful oxidizer. Combined with a fuel, it can be used as a rocket propellant called ammonium perchlorate composite propellant. Its instability has involved it in a number of accidents, such as the PEPCON disaster.

Perchloric acid is a mineral acid with the formula HClO4. It is an oxoacid of chlorine. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid, nitric acid and hydrochloric acid. It is a powerful oxidizer when hot, but aqueous solutions up to approximately 70% by weight at room temperature are generally safe, only showing strong acid features and no oxidizing properties. Perchloric acid is useful for preparing perchlorate salts, especially ammonium perchlorate, an important rocket fuel component. Perchloric acid is dangerously corrosive and readily forms potentially explosive mixtures.
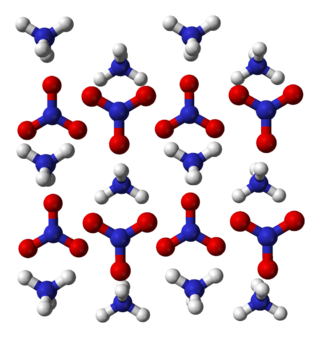
Ammonium nitrate is a chemical compound with the formula NH4NO3. It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, although it does not form hydrates. It is predominantly used in agriculture as a high-nitrogen fertilizer.
A propellant is a mass that is expelled or expanded in such a way as to create a thrust or another motive force in accordance with Newton's third law of motion, and "propel" a vehicle, projectile, or fluid payload. In vehicles, the engine that expels the propellant is called a reaction engine. Although technically a propellant is the reaction mass used to create thrust, the term "propellant" is often used to describe a substance which contains both the reaction mass and the fuel that holds the energy used to accelerate the reaction mass. For example, the term "propellant" is often used in chemical rocket design to describe a combined fuel/propellant, although the propellants should not be confused with the fuel that is used by an engine to produce the energy that expels the propellant. Even though the byproducts of substances used as fuel are also often used as a reaction mass to create the thrust, such as with a chemical rocket engine, propellant and fuel are two distinct concepts.

Dinitrogen pentoxide is the chemical compound with the formula N2O5. It is one of the binary nitrogen oxides, a family of compounds that contain only nitrogen and oxygen. It exists as colourless crystals that sublime slightly above room temperature, yielding a colorless gas.

A perchlorate is a chemical compound containing the perchlorate ion, ClO−4, the conjugate base of perchloric acid. As counterions, there can be metal cations, quaternary ammonium cations or other ions, for example, nitronium cation.
Rocket candy, or R-Candy, is a type of rocket propellant for model rockets made with a form of sugar as a fuel, and containing an oxidizer. The propellant can be divided into three groups of components: the fuel, the oxidizer, and the (optional) additive(s). In the past, sucrose was most commonly used as fuel. Modern formulations most commonly use sorbitol for its ease of production. The most common oxidizer is potassium nitrate (KNO3). Potassium nitrate is most commonly found in tree stump remover. Additives can be many different substances, and either act as catalysts or enhance the aesthetics of the liftoff or flight. A traditional sugar propellant formulation is typically prepared in a 65:35 (13:7) oxidizer to fuel ratio. This ratio can vary from fuel to fuel based on the rate of burn, timing and use.
A pyrotechnic composition is a substance or mixture of substances designed to produce an effect by heat, light, sound, gas/smoke or a combination of these, as a result of non-detonative self-sustaining exothermic chemical reactions. Pyrotechnic substances do not rely on oxygen from external sources to sustain the reaction.
Ammonium perchlorate composite propellant (APCP) is a solid rocket propellant. It differs from many traditional solid rocket propellants such as black powder or zinc-sulfur, not only in chemical composition and overall performance but also by being cast into shape, as opposed to powder pressing as with black powder. This provides manufacturing regularity and repeatability, which are necessary requirements for use in the aerospace industry.
In pyrotechnics, a pyrotechnic initiator is a device containing a pyrotechnic composition used primarily to ignite other, more difficult-to-ignite materials, such as thermites, gas generators, and solid-fuel rockets. The name is often used also for the compositions themselves.

Ammonium dinitramide (ADN) is an inorganic compound with the chemical formula [NH4][N(NO2)2]. It is the ammonium salt of dinitraminic acid HN(NO2)2. It consists of ammonium cations [NH4]+ and dinitramide anions −N(NO2)2. ADN decomposes under heat to leave only nitrogen, oxygen, and water.

A plastisol is a colloidal dispersion of small polymer particles, usually polyvinyl chloride (PVC), in a liquid plasticizer. When heated to around 180 °C (356 °F), the plastic particles absorb the plasticizer, causing them to swell and fuse together forming a viscous gel. Once this is cooled to below 60 °C (140 °F) it becomes a flexible, permanently plasticized solid product. This process is called 'curing'.

Rocket propellant is used as reaction mass ejected from a rocket engine to produce thrust. The energy required can either come from the propellants themselves, as with a chemical rocket, or from an external source, as with ion engines.
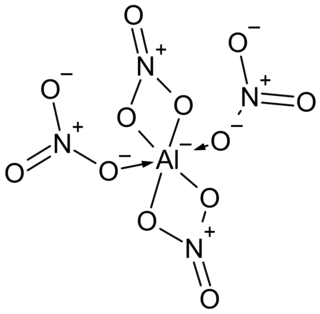
Tetranitratoaluminate is an anion of aluminium and nitrate groups with formula [Al(NO3)4]− that can form salts called tetranitratoaluminates. It is unusual in being a nitrate complex of a light element.
Hexanitratoaluminate is an anion of aluminium and six nitrate groups with formula [Al(NO3)6]3− that can form salts called hexanitratoaluminates.
The hexaperchloratoaluminate ion is a polyatomic anion with the chemical formula [Al(ClO4)6]3−. It is composed of six perchlorate (ClO−4) anions bound to a central aluminium ion (Al3+), resulting in a net charge of –3. This ion is a highly oxidizing and reactive complex, similar to other hexacoordinate aluminium complexes such as hexanitratoaluminate.
Strontium perchlorate is a white powder or colorless crystals with the formula Sr(ClO4)2.

Nitrosyl perchlorate is the inorganic compound with the formula NO(ClO4). A hygroscopic white solid, it is the salt of the nitrosonium cation with the perchlorate anion. It is an oxidant and strong electrophile, but has fallen out of use with the availability of the closely related salt nitrosonium tetrafluoroborate NO(BF4).












