
An explosive is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An explosive charge is a measured quantity of explosive material, which may either be composed solely of one ingredient or be a mixture containing at least two substances.
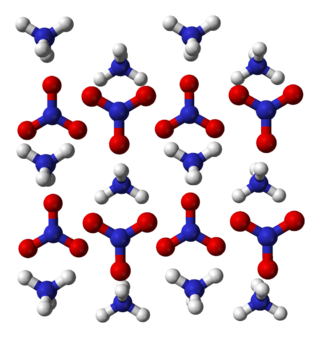
Ammonium nitrate is a chemical compound with the formula NH4NO3. It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, although it does not form hydrates. It is predominantly used in agriculture as a high-nitrogen fertilizer.

In physics, a shock wave, or shock, is a type of propagating disturbance that moves faster than the local speed of sound in the medium. Like an ordinary wave, a shock wave carries energy and can propagate through a medium but is characterized by an abrupt, nearly discontinuous, change in pressure, temperature, and density of the medium.
In spark-ignition internal combustion engines, knocking occurs when combustion of some of the air/fuel mixture in the cylinder does not result from propagation of the flame front ignited by the spark plug, but when one or more pockets of air/fuel mixture explode outside the envelope of the normal combustion front. The fuel–air charge is meant to be ignited by the spark plug only, and at a precise point in the piston's stroke. Knock occurs when the peak of the combustion process no longer occurs at the optimum moment for the four-stroke cycle. The shock wave creates the characteristic metallic "pinging" sound, and cylinder pressure increases dramatically. Effects of engine knocking range from inconsequential to completely destructive.
A pulse detonation engine (PDE) is a type of propulsion system that uses detonation waves to combust the fuel and oxidizer mixture.

Deflagration is subsonic combustion in which a pre-mixed flame propagates through an explosive or a mixture of fuel and oxidizer. Deflagrations in high and low explosives or fuel–oxidizer mixtures may transition to a detonation depending upon confinement and other factors. Most fires found in daily life are diffusion flames. Deflagrations with flame speeds in the range of 1 m/s differ from detonations which propagate supersonically with detonation velocities in the range of km/s.

The shock tube is an instrument used to replicate and direct blast waves at a sensor or a model in order to simulate actual explosions and their effects, usually on a smaller scale. Shock tubes can also be used to study aerodynamic flow under a wide range of temperatures and pressures that are difficult to obtain in other types of testing facilities. Shock tubes are also used to investigate compressible flow phenomena and gas phase combustion reactions. More recently, shock tubes have been used in biomedical research to study how biological specimens are affected by blast waves.
RDS-37 was the Soviet Union's first two-stage hydrogen bomb, first tested on 22 November 1955. The weapon had a nominal yield of approximately 3 megatons. It was scaled down to 1.6 megatons for the live test.
Explosive velocity, also known as detonation velocity or velocity of detonation (VoD), is the velocity at which the shock wave front travels through a detonated explosive. Explosive velocities are always faster than the local speed of sound in the material.

A valveless pulsejet is the simplest known jet propulsion device. Valveless pulsejets are low in cost, light weight, powerful and easy to operate. They have all the advantages of conventional valved pulsejets, but without the reed valves that need frequent replacement; a valveless pulsejet can operate for its entire useful life with practically zero maintenance. They have been used to power model aircraft, experimental go-karts, and unmanned military aircraft such as cruise missiles and target drones.

The Chapman–Jouguet condition holds approximately in detonation waves in high explosives. It states that the detonation propagates at a velocity at which the reacting gases just reach sonic velocity as the reaction ceases.
The Richtmyer–Meshkov instability (RMI) occurs when two fluids of different density are impulsively accelerated. Normally this is by the passage of a shock wave. The development of the instability begins with small amplitude perturbations which initially grow linearly with time. This is followed by a nonlinear regime with bubbles appearing in the case of a light fluid penetrating a heavy fluid, and with spikes appearing in the case of a heavy fluid penetrating a light fluid. A chaotic regime eventually is reached and the two fluids mix. This instability can be considered the impulsive-acceleration limit of the Rayleigh–Taylor instability.

A flame arrester, deflagration arrester, or flame trap is a device or form of construction that will allow free passage of a gas or gaseous mixture but will interrupt or prevent the passage of flame. It prevents the transmission of flame through a flammable gas/air mixture by quenching the flame on the high surface area provided by an array of small passages through which the flame must pass. The emerging gases are cooled enough to prevent ignition on the protected side.

An explosion is a rapid expansion in volume of a given amount of matter associated with an extreme outward release of energy, usually with the generation of high temperatures and release of high-pressure gases. Explosions may also be generated by a slower expansion that would normally not be forceful, but is not allowed to expand, so that when whatever is containing the expansion is broken by the pressure that builds as the matter inside tries to expand, the matter expands forcefully. An example of this is a volcanic eruption created by the expansion of magma in a magma chamber as it rises to the surface. Supersonic explosions created by high explosives are known as detonations and travel through shock waves. Subsonic explosions are created by low explosives through a slower combustion process known as deflagration.
Deflagration to detonation transition (DDT) refers to a phenomenon in ignitable mixtures of a flammable gas and air when a sudden transition takes place from a deflagration type of combustion to a detonation type of explosion.
The ZND detonation model is a one-dimensional model for the process of detonation of an explosive. It was proposed during World War II independently by Y. B. Zel'dovich, John von Neumann, and Werner Döring, hence the name.
Jacques Charles Émile Jouguet was a French engineer and scientist, whose name is attached to the Chapman–Jouguet condition.

The Fickett–Jacobs cycle is a conceptual thermodynamic cycle that allows to compute an upper limit to the amount of mechanical work obtained from a cycle using an unsteady detonation process (explosive). The Fickett–Jacobs (FJ) cycle is based on Chapman–Jouguet (CJ) theory, an approximation for the detonation wave's velocity during a detonation. This cycle is researched for rotating detonation engines (RDE), considered to be more efficient than the classical combustion engines that are based on the Brayton or Humphrey cycles.
A Zeldovich spontaneous wave, also referred to as Zeldovich gradient mechanism, is a reaction wave that propagates spontaneously in a reacting medium with a nonuniform initial temperature distribution when there is no interaction between different fluid elements. The concept was put forward by Yakov Zeldovich in 1980, based on his earlier work with his coworkers. The spontaneous wave is different from the other two conventional combustion waves, namely the subsonic deflagrations and supersonic detonations. The wave, although strictly speaking unrealistic because gasdynamic effects are neglected, is often cited to explain the yet-unsolved problem of deflagration to detonation transition (DDT).

Pressure gain combustion (PGC) is the unsteady state process used in gas turbines in which gas expansion caused by heat release is constrained. First developed in the early 20th century as one of the earliest gas turbine designs, the concept was mostly abandoned following the advent of isobaric jet engines in WWII.













